Therapeutic Strategies for Alpha-1 Antitrypsin Deficiency
What is alpha-1 antitrypsin deficiency?
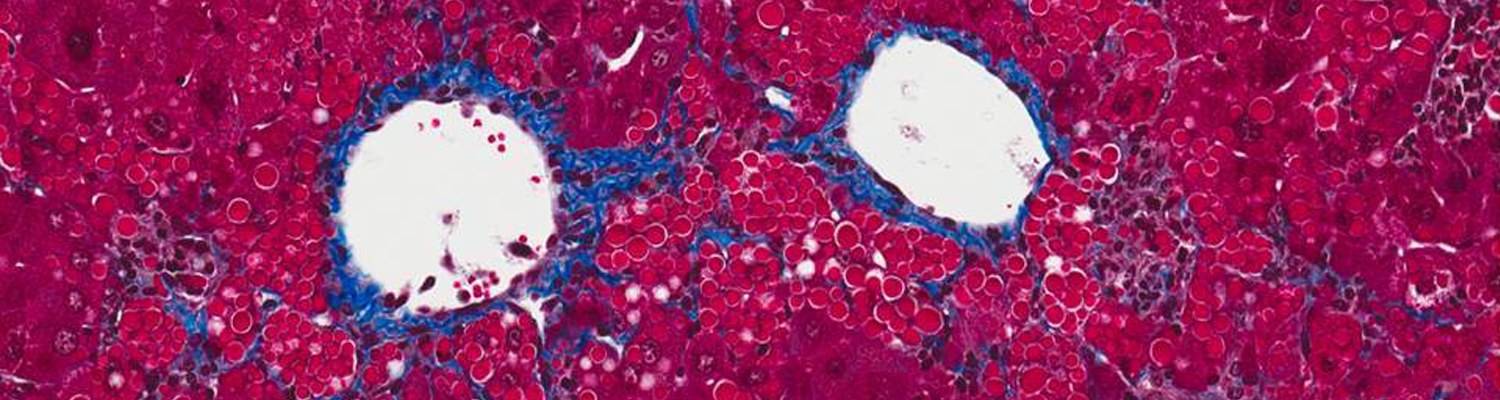
Alpha-1 antitrypsin (AAT) deficiency is a common (although often unrecognized) genetic disease, with up to 4% of the population carrying an abnormal AAT gene. Patients can suffer from either a lung disease, which is caused by a loss of the normal AAT action, or from a liver disease, which is caused by build-up of the abnormal mutant protein (called Z-AAT) within the liver cells.
AAT deficiency occurs worldwide, but the prevalence is variable depending on regions. It is a rare disease in Europe and in the US. It affects between 1:1500 and 1:3500 in people of European descent. It is the 2nd most common genetic disorder in Ireland, where 1:25 people carry the mutant gene. It is rare in Asians.
Developing AAT therapeutics
The laboratories of Drs. Terry Flotte, Guanping Guo,Chris Mueller, Wen Xue, and Mai ElMallah are designing gene therapies to treat the lung disease by replacing the normal AAT protein and now is using a combined gene therapy-RNA interference (RNAi) approach to test a potential treatment for the liver disease. Initial efforts used a gene therapy agent called rAAV8 to deliver a form of interfering RNA called a short-hairpin (shRNA). These studies showed a 50% reduction of the offending mutant Z-AAT protein in a mouse. Subsequently, we have developed potentially less toxic approaches based on incorporating the natural microRNA structure into the short hairpin within the rAAV8 gene therapy agent. These microRNAs were altered to allow them to block production of the toxic Z-AAT. Early results from experiments in mice show that the new strategy reduces the toxic Z-AAT by as much as 80%. We have also designed gene therapy agents that should allow us to simultaneous block production of the toxic Z-AAT and make the healthy AAT protein within the same liver cells. Our immediate goal is to improve the effectiveness of this strategy in mice, while ensuring its safety, with the long term goal of developing these methods into therapy for treating AAT deficient patients.
Five-year, $11 million grant to fund new approaches to gene therapy for AAT