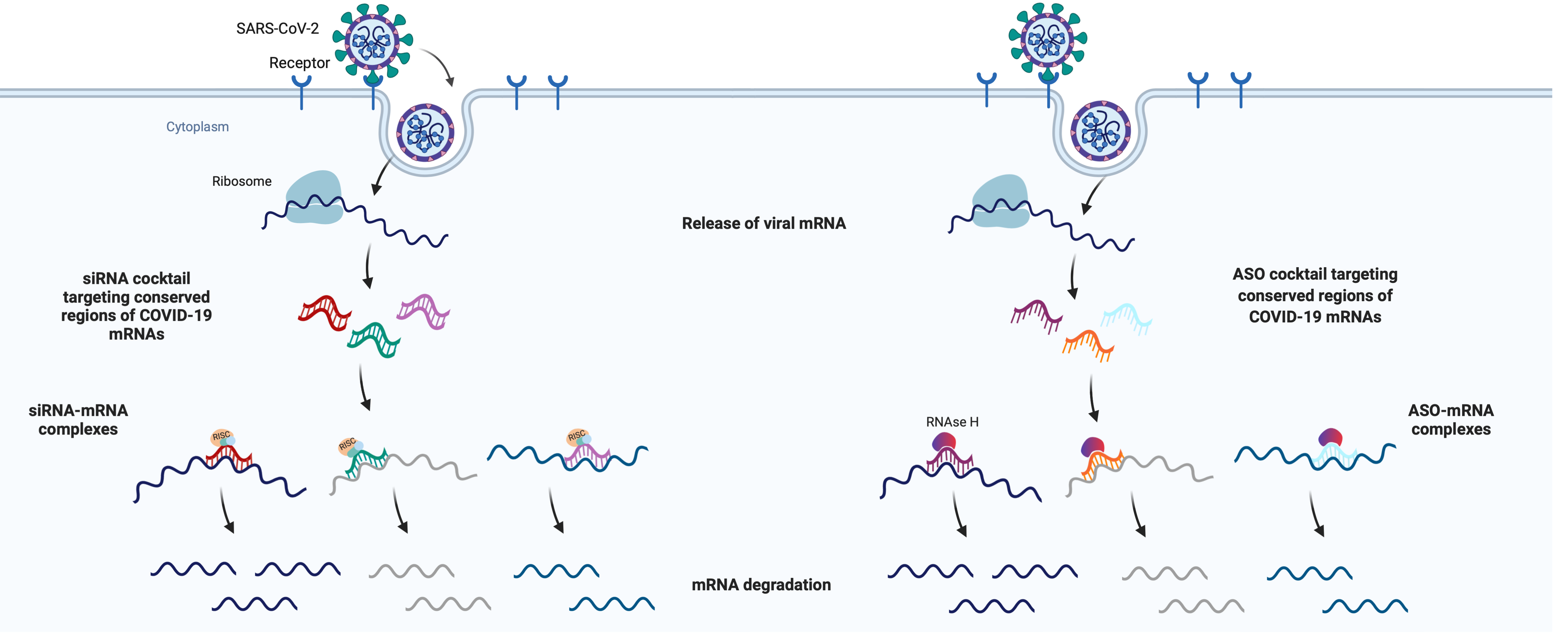
COVID-19 Therapeutic Development in the RTI
This is your opportunity to take an active role in the fight against coronavirus.
Every dollar you donate goes directly to UMass Chan labs working on front-line research for treatments, tests and vaccines. Please ... won't you join us?