An S-phase –dependent cell fate decision
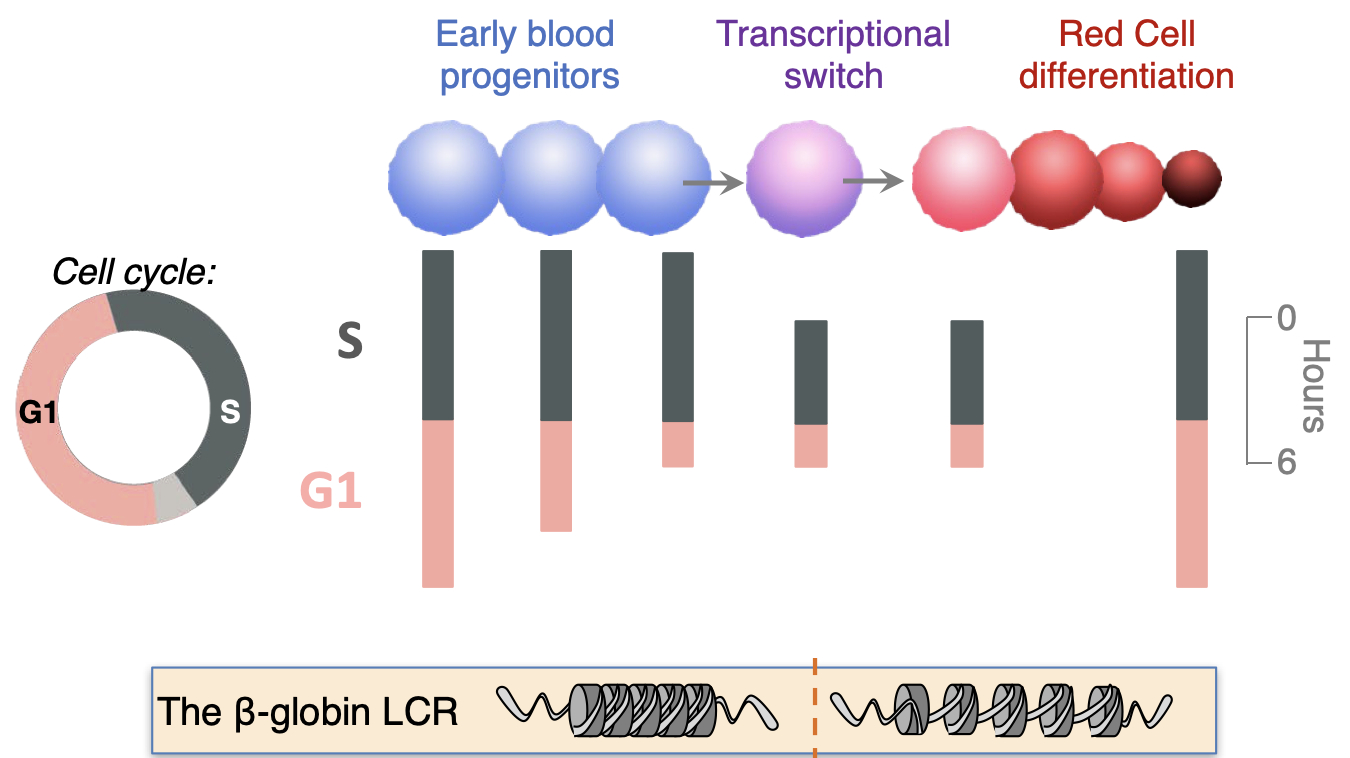
We discovered that early erythroid progenitors known as 'colony-forming-unit-erythroid' (CFU-e) transition from a state of self-renewal to erythroid terminal differentiation (ETD) during a rapid transcriptional switch that activates expression of erythroid genes such as hemoglobin genes. The CFU-e/ ETD switch is synchronized with, and dependent on, S phase progression. This specific S phase is unexpectedly shorter and faster than that of preceding or ensuing cycles, propelled by globally-faster replication forks, a novel mechanism in the physiological regulation of S phase length. Using single-cell RNA-sequencing, we recently confirmed the synchrony between the CFU-e/ ETD cell fate switch and S phase, at the single-cell transcriptional level.
Further, in addition to S phase shortening at the time of the CFU-e/ ETD switch, there is gradual shortening of G1 phase of the cycle during the period that leads up to the switch. We have identified a number of regulators that coordinate these dramatic cell cycle remodeling events in lock-step with erythroid developmental decisions, including the erythropoietin receptor (EpoR), the cell cycle regulator p57KIP2, and the transcription factor PU.1.
References:
Pop, R., Shearstone, J.R., Shen, Q., Liu, Y., Hallstrom, K., Koulnis, M., Gribnau, J., and Socolovsky, M. (2010). A key commitment step in erythropoiesis is synchronized with the cell cycle clock through mutual inhibition between PU.1 and S-phase progression. PLoS Biol 8.
Shearstone, J.R., Pop, R., Bock, C., Boyle, P., Meissner, A., and Socolovsky, M. (2011). Global DNA demethylation during mouse erythropoiesis in vivo. Science 334, 799-802.
Hwang Y, Futran M, Hidalgo D, Pop R, Iyer DR, Scully R, Rhind N, Socolovsky M. Global increase in replication fork speed during a p57KIP2-regulated erythroid cell fate switch. Sci Adv. 2017 May 26;3(5):e1700298. doi: 10.1126/sciadv.1700298. PMID: 28560351; PMCID: PMC5446218.
Tusi BK, Wolock SL, Weinreb C, Hwang Y, Hidalgo D, Zilionis R, Waisman A, Huh JR, Klein AM, Socolovsky M. Population snapshots predict early haematopoietic and erythroid hierarchies. Nature. 2018 Mar 1;555(7694):54-60. doi: 10.1038/nature25741. Epub 2018 Feb 21. PMID: 29466336; PMCID: PMC5899604
Daniel Hidalgo, Jacob Bejder, Ramona Pop, Kyle Gellatly, Yung Hwang, S. Maxwell Scalf, Anna E. Eastman, Jane-Jane-Chen, L.Julie Zhu, Jules AAC Heugerger, Shangqin Guo, Mark J. Koury, Nikolai Baastrup Nordsborg, Merav Socolovsky. EpoR stimulates rapid cycling and larger red cells during mouse and human erythropoiesis. Nature Communications 2021 Dec 17; 12(1):7334. https://doi.org/10.1038/s41467-021-27562-4 PMID: 34921133
