Investigator Spotlight, Sandra Almeida, PhD
 Preventing innocent cells from making villain proteins is a focus of Sandra Almeida, PhD. She seeks to understand how mutations in specific genes cause amyotrophic lateral sclerosis and frontotemporal dementia (ALS/FTD). In March of 2023, Dr. Almeida co-authored a review article in Gene titled, “How villains are made: The translation of repeat proteins in C9ORF72-ALS/FTD.” Almeida discussed the mechanisms responsible for the toxic RNA and proteins which inspired her research to establish elimination strategies to remove villains found in the C9ORF72 gene.
Preventing innocent cells from making villain proteins is a focus of Sandra Almeida, PhD. She seeks to understand how mutations in specific genes cause amyotrophic lateral sclerosis and frontotemporal dementia (ALS/FTD). In March of 2023, Dr. Almeida co-authored a review article in Gene titled, “How villains are made: The translation of repeat proteins in C9ORF72-ALS/FTD.” Almeida discussed the mechanisms responsible for the toxic RNA and proteins which inspired her research to establish elimination strategies to remove villains found in the C9ORF72 gene.
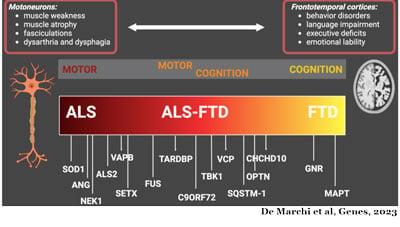
Sandra Almeida, PhD, is a scientist, and assistant professor in the Department of Neurology at UMass Chan. Dr. Almeida began studying FTD in 2008 as part of her post-doctoral studies in the laboratory of Fen-Biao Gao at the University of California, San Francisco (UCSF) before joining UMass Chan in 2010. Her research interests expanded to include the ALS/FTD continuum in 2011 when the most common genetic mutation associated with the disease was found in the C9ORF72 gene.
This gene produces the C9ORF72 protein that plays a role in the autophagy pathway, the cell’s recycling system. When the mutation is present in the cell’s DNA, the cell produces insufficient amounts of the healthy C9ORF72 protein affecting its ability to recycle its waste materials. Additionally, the RNAs containing the mutation, a large repetition of the same six units, produce harmful proteins called dipeptide repeat proteins (DPR), and/or work as decoys and dismantle the normal cell processes.
The Almeida Lab studies the production of DPR proteins within cells using human neurons. These neurons are derived from induced pluripotent stem cells (iPSCs), which are obtained by modifying skin cells from individuals with ALS/FTD due to the C9ORF72 mutation. By examining the initiation codon sequence in RNA, she aims to uncover the source of DPR proteins. Dr. Almeida shares, "Once we have this information, we can devise strategies to disrupt the production of these abnormal proteins and hopefully this will help to slow down disease progression.”
Altering the recipe of toxic proteins is not a straightforward process because these instructions are embedded in the body’s DNA and modifying them can impact their chemistry and the way they interact and function. Thus, she aims to develop strategies to reduce or eliminate the toxic threat the DPR proteins create in the body. T discovery has brought forth important unanswered questions: When do things go wrong? How long can these toxic threats live in the body without creating harm, and when do symptoms begin to develop?
Her research centers on understanding the disease composition, when the processes initiated by the mutation start, and considering all mechanisms happening in the process. She is driven to understand the early translation of symptoms to develop treatment strategies before the disease progresses to prolong a patient’s life span and quality of life.
In October of 2023, the Almeida Lab received a grant from the National Institute of Aging (NIA) to develop an iPSC-derived neurovascular unit to study the different causes leading to the loss of the progranulin protein contributes to FTD. This endeavor will also include developing a platform model to identify and characterize disease-relevant phenotypes.
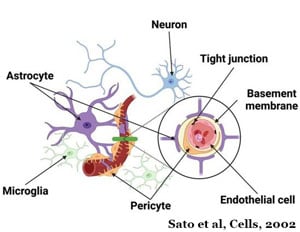 Mutations in the Granulin gene are a primary cause of the progranulin protein which is a major driver of FTD. Like the C9ORF72 protein, progranulin also works in the cell's recycling system. When the mutation is present, the cell either recycles the instructions for making the progranulin protein or makes a faulty protein.
Mutations in the Granulin gene are a primary cause of the progranulin protein which is a major driver of FTD. Like the C9ORF72 protein, progranulin also works in the cell's recycling system. When the mutation is present, the cell either recycles the instructions for making the progranulin protein or makes a faulty protein.
This funding will also allow her to devise ways to reverse the effects of the loss of the progranulin protein. She will seek methods to boost progranulin levels in human cells relevant to FTD restoring them to health to delay disease progression and neurodegeneration. Because loss of the progranulin protein also increases the risk for other neurodegenerative diseases, including Alzheimer’s disease, augmenting progranulin in brain cells can potentially help people with FTD, as well as others affected by neurological disorders that are currently incurable.