Retinal Degeneration
What is Retinal Degeneration?
Blindness is the inevitable end stage of retinal degeneration, meaning the irreversible loss of neurons in the retina. The two cell types in the retina that are generally associated with loss of vision in humans are either the ganglion cells or the photoreceptors. Loss of ganglion cells results in Glaucoma, a devastating disease affecting ~ 3 Millions in the United States alone. Loss PRs is associated with a large number of retinal degenerative diseases of which Age-related-Macular-Degeneration (AMD) is the most widespread with over 2 millions affected in the United States. Since photoreceptors account for ~75% of all cells in the retina, loss of photoreceptors results always in sever retinal degeneration.
Lab Interest in Photoreceptor Degeneration
The research focus in my group is on retinal degenerative diseases that affect photoreceptors, in particular cone photoreceptors, because cones are essential for color, daylight and high acuity vision in humans. One peculiarity about the human and mouse retina is that loss of rod photoreceptors always trigger loss of cones. In contrast, loss of cones has no effect on rods. This interesting fundamental biological question has very important implication with regards to vision loss in humans. For example, Retinitis Pigmentosa is a family of inherited retinal degenerations that is untreatable and leads to blindness. In many cases, the disease-causing mutation is in a gene that is exclusively expressed in rod photoreceptors. However, because rod loss always leads to cone loss cones die too. Since rods are generally affected first in Retinitis Pigmentosa the pathology is characterized by an initial loss of night vision, due to the loss of rod photoreceptors, followed by a progressive loss peripheral vision as cones die, until only the macular cones remain. At this stage of the disease individuals affected by Retinitis Pigmentosa experience tunnel vision, until eventually they lose complete sight. Understanding this non-autonomous cone death is the key in designing therapeutic strategies for retinal degenerative diseases such as Retinitis Pigmentosa. While the dependence of cones on the presence of rods plays an important role in Retinitis Pigmentosa, it remains a fundamental question of retinal biology.
Why do Cones die in Retinitis Pigmentosa
 |
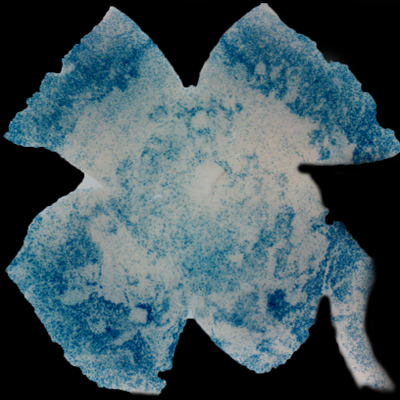 |
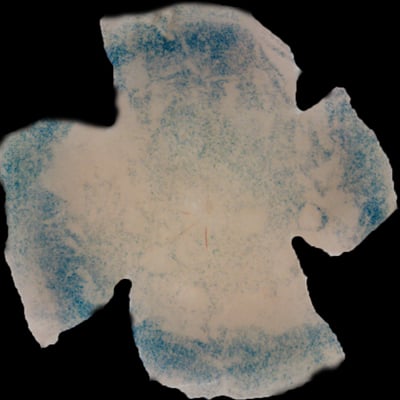
| Insulin affects cone survival in Retinitis Pigmentosa. Shown is a retinal flat mount of an rd1 mutant showing surviving cones at 7 weeks of age in blue (left panel). The rd1 mutant is part of the Retinitis Pigmentosa family of RD diseases. Rollover image to see normal cone distribution at 3 weeks of age in the same mutant before the onset of cone death. To the right is an image of a retinal flat mount at 7 weeks of age of an rd1 mutant treated for 4 weeks with daily injections of insulin showing an increased number of surviving cones. Rollover image to see what happens when endogenous insulin is removed for the same period of time. | |
Our recent studies on Retinitis Pigementosa have led us to propose that cone death is preceded by metabolic changes in cone photoreceptors and that cones die due to nutrient deprivation, in particular glucose. This hypothesis was based on gene expression changes seen at the onset of cone death in many gene that are involved in cell metabolism in addition to gene expression changes in genes that belong to the Insulin/mTOR signaling pathway. This signaling pathway is a key pathway in regulating cellular metabolism. As a proof of concept we injected mice systemically with insulin for a period of 4 weeks to test if stimulation of the pathway could improve cone survival (Figure to the right). The positive outcome of our insulin treatment reinforced our hypothesis. However, contrary to the regular action of insulin in muscle, liver and adipose tissue, where it enhances glucose uptake from the blood by release of the glucose transporter 4 to the cell membrane, the action of insulin on the retina improved glucose metabolism by changing the expression of various glycolytic genes that are regulated by the insulin/mTOR pathway.
Why do Cone Photoreceptors depend on Rod photoreceptors?
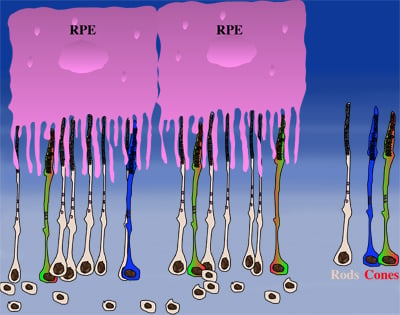
How could the observations of nutritionally deprived cones explain the dependence of cones on rods? Photoreceptor outer segments interact with the retinal-pigmented epithelium, which a single sheet of cells adjacent to the photoreceptor layer. The outer segments-retinal-pigmented epithelium interactions are vital since the retinal-pigmented epithelium provides nutrition and oxygen to PRs. Roughly 95% of all PRs in mouse and human are rods and approximately 20-30 outer segments contact one retinal-pigmented epithelium cell. Thus, only 1-2 of those retinal-pigmented epithelium-outer segment contacts are via cones. In Retinitis Pigmentosa rods die primarily due to a mutation in a rod specific gene. The loss of rods, which comprise ~95% of all photoreceptors leads to a collapse of the outer nuclear layer. During this collapse, the few remaining cone:retinal-pigmented epithelium interactions are likely perturbed (Rollover image below). If these interactions drop below a critical threshold required for the proper flow of nutrients, the loss of rods results in reduced flow of nutrients to cones. By cross-comparing 4 mouse models of Retinitis Pigmentosa we found in our studies that cone death starts always after ~90% of rods have died. This cell density thus represent the crucial threshold of remaining cells after which flow of nutrition is perturbed. This mechanism also explains why the loss of cones does not lead to rod death. Since in humans and mouse, cones are less than 5% of all PRs, the critical threshold that perturbs outer segment-retinal-pigmented epithelium interactions is not be reached. Additionally, it also explains why in cone rich species such as Zebrafish loss of cones does affect rod survival. In summary, the determining factor for the less abundant photoreceptor cell type to die when the more abundant one dies, is the local ratio between the 2 photoreceptor cell types.
 |
 |
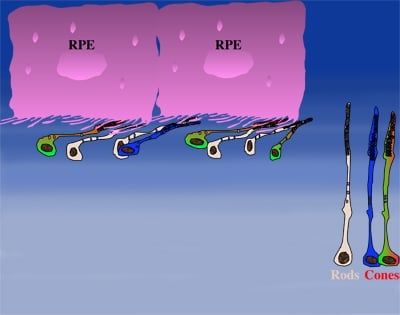 |
| During rod cell death the cell density decreases in the outer nuclear layer (rollover first panel to see the number of rods decrease). The reduced number of rods in the outer nuclear layer results in a collapse of the ONL and put stress on the outer segment retinal-pigmented epithelium interactions (rollover second panel to detach the outer segments from the retinal-pigmented epithelium). At later stages of degeneration a retinal cross-section looks similar to what is seen in the last panel. Once this critical threshold of remaining cells is breached cones start to die due to reduced nutrient flow while the remaining rods die due to the intrinsic mutation. | ||
