Research
CEP290 and Leber's congenital amaurosis
Mutations in CEP290 are the most frequently identified cause of Leber’s congenital amaurosis (LCA), a severe, early-onset blindness. We’re using both Chlamydomonas and mouse CEP290 mutants to elucidate the molecular function of CEP290. We’ve found that CEP290 localizes to the transition zone of the Chlamydomonas flagellum. High resolution immuno-EM, carried out in collaboration with Joel Rosenbaum's lab at Yale University, further revealed that CEP290 is closely associated with the highly conserved Y-shaped microtubule-membrane connectors that are characteristic of the transition zone in many organisms. In the absence of CEP290, these linkers are missing or disrupted. Taken together, the results strongly suggest that CEP290 is a component of the linkers. Flagella that lack CEP290 have an abnormal protein composition, including abnormal levels of proteins such as polycystin-2 and BBS4, which are involved in polycystic kidney disease and Bardet-Biedl syndrome, respectively; this may account for the high degree of phenotypic overlap between CEP290 patients and patients with the latter two disorders. We also found that CEP290 is dynamic and rapidly exchanges at the transition zone, which has positive implications for the prospect of successful gene therapy in the retinas of patients with LCA. In the mouse, we found that CEP290 is localized to the photoreceptor cell's connecting cilium, which is structurally and functionally analogous to the transition zone of Chlamydomonas. Our working model is that CEP290 and the Y-shaped linkers at the transition zone form a gate or quality control machinery that regulates entry of proteins into the ciliary compartment.
Localization and function of nephrocystin-4 in Chlamydomonas
|
|
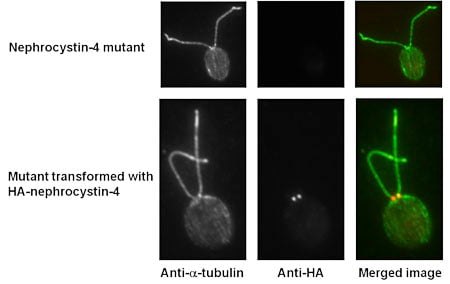
| Immunofluorescence micrographs of the nephrocystin-4 null mutant before and after transformation with nephrocystin-4-3XHA. Cells are labeled with an antibody to alpha-tubulin to reveal microtubules in the cell body and flagella, and an antibody to the HA epitope to reveal the location of nephrocystin-4-3XHA in the flagellar transition zone. In the merged image, tubulin is labeled green and nephrocystin-4-3XHA is labeled red. |
Mutations in human NPHP4, encoding nephrocystin-4, cause nephronophthisis (kidney disease) and retinitis pigmentosa (blindness). We are using Chlamydomonas as a model system to learn more about the function of nephrocystin-4 and why defects in it cause human disease. Using immunofluorescence microscopy with antibodies against Chlamydomonas nephrocystin-4, we found that the protein is located at the base of the flagellum. The location of nephrocystin-4 is conserved among species, suggesting that its function also has been conserved. Immuno-gold electron microscopy in which Chlamydomonas cells expressing HA-tagged nephrocystin-4 were probed with antibodies against the HA peptide indicate that nephrocystin-4-HA is localized specifically to the transition zone. A Chlamydomonas insertional mutant that is null for nephrocystin-4 has normal length flagella, so the protein is not essential for flagellar assembly. Moreover, SDS-PAGE indicates that the protein compositions of wild-type and mutant axonemes are nearly identical. However, the protein compositions of wild-type and mutant membrane-plus-matrix fractions are quite different. Identification of the proteins that are specifically altered in the mutant will help elucidate the specific function of nephrocystin-4.
The Chlamydomonas flagellar membrane lipidome
Chlamydomonas has been extensively investigated as a model for the role of cilia in human health and disease. However, almost all studies of flagella have focused exclusively on proteins with no attention to lipids. Because lipids are likely to be important in sorting of flagellar proteins in the trans-Golgi network, in intraflagellar transport, and in the function of cilia, we are carrying out a project to establish the lipidome of the Chlamydomonas flagellum. The lipid profile of whole cells, cell bodies, flagella, and the cell body plasma membrane are being compared by mass spectrometry. The analyses reveal that flagella, cell bodies, and the plasma membrane all differ in their lipid and fatty acid profiles. Our findings suggest for the first time that the flagellar membrane of Chlamydomonas has a lipid and fatty acid composition distinct from that of the plasma membrane with which it is ultrastructurally continuous.
Generation and analysis of new mutants informative about flagellar assembly and function
A major advantage of Chlamydomonas has been the ability to generate insertional mutants for the study of flagellar proteins. Such mutants have been key to many of our most important papers over the past 15 years, and have been largely responsible for the progress of the field in general. Recently, we have developed a method that greatly facilitates this approach by allowing the genomic sequence at both ends of an insert to be quickly determined, so that the disrupted gene is immediately known. The method involves insertional mutagenesis using a selectable marker (pHyg3) followed by screening for mutants with defects in flagellar assembly or function. An optimized restriction enzyme site-directed amplification PCR (RESDA PCR) protocol is then used to analyze the mutants to determine the flanking DNA at both ends of the insert. The screening and PCR is carried out in a 96-well format and so is readily adapted to high-throughput methodology. The mutant can then be characterized structurally and biochemically to understand the function of the mutated gene.
Regulation of expression of genes encoding ciliary proteins
Despite the importance of cilia for human development and the connection between ciliary defects and human disease, little is known about the regulatory networks controlling expression of genes encoding ciliary proteins. We are taking a genetic approach using Chlamydomonas as a model system to learn more about this important process. Deflagellation of Chlamydomonas results in rapid and coordinate induction of hundreds of genes, and the resulting new protein synthesis is required for regeneration of flagella, which are essentially identical to cilia. We have developed a reporter strain in which expression of a Gaussia princeps luciferase is driven by the promoter for dynein light chain LC8, an abundant flagellar protein upregulated by deflagellation. Deflagellation of this strain results in an increase in luciferase activity similar to increases in expression of genes encoding flagellar proteins. This has allowed us to screen for insertional mutants in the reporter background that are unable induce luciferase. Two mutants identified so far have abnormal flagellar regeneration kinetics, suggesting that the mutagenized genes may regulate more than just the LC8 gene. Further screening and characterization of mutants is underway.