Thompson Lab's Mitesh Nagar published paper in Frontiers in Immunology
Date Posted: Thursday, March 21, 2019
Thioredoxin Modulates Protein Arginine Deiminase 4 (PAD4)-Catalyzed Citrullination
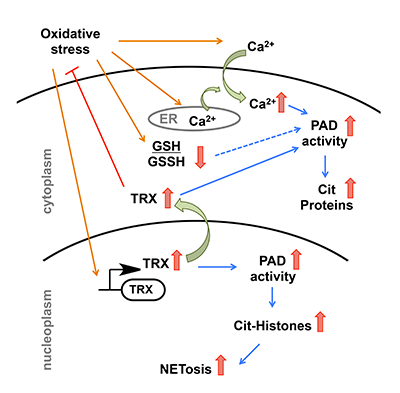
Protein arginine deiminases (PADs) convert protein bounds arginines into a citrulline residue. Since the modifications catalyzed by the PADs alter the charge on the proteins, these enzymes perform a variety of physiological functions including structural support and gene-regulation. However, uncontrolled activity of PADs can do more harm than good as shown by a plethora of literature that links aberrant PAD activity with several autoimmune disorders including rheumatoid arthritis and various form of cancer. Biochemical events that trigger PAD activity to switch from physiological to pathophysiological are still unclear. Recently, Dr. Nagar from the Thompson lab published “Thioredoxin Modulates Protein Arginine Deiminase 4 (PAD4)-Catalyzed Citrullination” in Frontiers in Immunology. This publication has already been read 800 time with greater than 250 downloads in a short time frame. In this paper, Dr. Nagar showed that thioredoxin, an important defense molecule against oxidative stress, increased the catalytic activity of PADs in a non-canonical manner. This recent work from Thompson lab also provides a direct link between higher thioredoxin levels and enhanced protein citrullination in RA patients, and further highlights how environmental factors that induce oxidative stress could lead to PAD activation within cells.
To read more about this exciting work click on the link below.
Pubmed ID: 30853960
For more about Dr. Paul Thompson: https://www.umassmed.edu/thompson
