Research
Our research is centered in understanding the morphogenetic processes and the molecules that guide the development of the mouse embryo from the one-cell stage to the initial stages of organogenesis. To achieve this goal, we manipulate gene expression in vivo, to elucidate gene function at the cellular and organismic level.
Mutations that affect early embryogenesis can lead to major morphological defects. Mice carrying
a mutation in one copy of Bachyury lack or have a shortened tail as a consequence of abnormal gastrulation.
Mouse development studies and human health
Why study early mammalian development? There are multiple reasons: It is known that thirty percent of all human pregnancies are lost at early post-implantation stages. Also, with the advent of assisted conception, the number of monozygotic twins has quadrupled, leading to an increase in perinatal mortality, pre-term birth and birth weight discordance. Research in early mammalian development is also important for embryonic stem cell research and tumorigenesis. In the case of embryonic stem cells, the strategies to produce specific and functional cell types for clinical applications require our understanding of lineage commitment and differentiation of embryonic cells in vivo. Early mammalian development also offers an excellent model for the study of epithelial to mesenchymal transition or EMT. EMT is a process in which epithelial cells are converted into motile cells, a pre-requisite for gastrulation. Unfortunately, EMT is also a pre-requisite for the metastasis of carcinomas. Hence understanding the process of gastrulation during early development is relevant not just for understanding basic mammalian development but also has implications in cancer research.

Olmec co-joined twins figurine. National Museum of Anthropology, Mexico City.
Specification of the primary embryo axes
One of the main goals of our laboratory is to understand how the primary axes of the embryo, the anteroposterior, dorsoventral and left/right axes are established during development. The proper generation of these axes is paramount for subsequent embryo development since they serve as the frame on which the rest of the embryo is constructed. We are currently studying the role of Wnt signaling in the establishment of the anteroposterior axis. Wnt signaling has been implicated in axial development of amphibians. Our hypothesis is that Wnt signaling, as in other vertebrates, is the major player in the establishment of the primary axes in mammals.
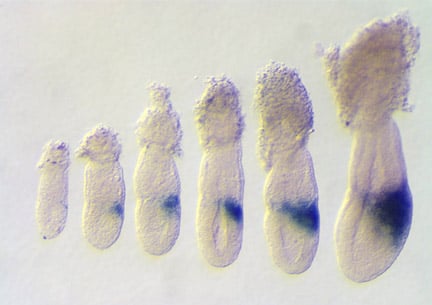
Expression of Wnt3 in embryos between 5.5 and 6.5 days of gestation. The expression of Wnt3 in the posterior visceral endoderm and subsequently posterior epiblast, marks the location and polarity of the anteroposterior axis. Rivera-Perez and Magnuson, 2005. Dev. Biol. 288:363.
Extra-embryonic tissues and early organogenesis
The mouse conceptus at early post-implantation stages is composed mostly of extra-embryonic tissues that give rise to the placenta and to the membranes that encircle the fetus. These extra-embryonic tissues provide nutrition, gas exchange and mechanical protection to the developing embryo allowing its survival inside the uterus. There is also evidence that extra-embryonic tissues provide patterning clues. We are interested in the development of the extra-embryonic tissues and in understanding the interactions between extra-embryonic and embryonic tissues that herald the initial stages of organogenesis.
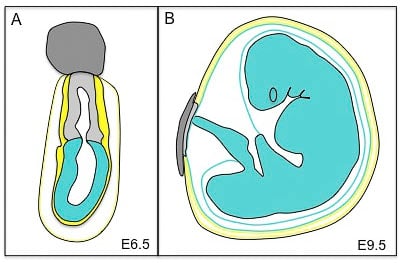
Mouse conceptus at embryonic day 6.5 (E6.5) and its derivatives at E9.5. At E6.5 the conceptus is composed of the ectoplacental cone (dark grey), the extra-embryonic ectoderm (light grey), the visceral endoderm (yellow) and the parietal yolk sac (black and yellow). The embryonic component of the conceptus is the epiblast (blue). At E9.5, the extra-embryonic tissues are composed of the placenta (dark and light grey) and the three membranes that surround the fetus: the amnion (an epiblast derivative, blue), the visceral yolk sac (blue and yellow) and the parietal yolk sac (yellow and black). The fetus is derived from the epiblast.
Lineage analysis and cell fate determination
Although the instructions for the development of the mammalian embryo reside in the genome, cell-cell interactions are pivotal to pattern an organism. A hallmark of our research is the use of clonal analysis in cell fate determination. We utilize a technique called iontophoresis to label individual cells in an embryo. Clonal analysis permits assessment of the position and nature of the descendants of a single labeled cell. These studies are fundamental for understanding how pluripotent cells choose a particular fate as the embryo progresses in development.
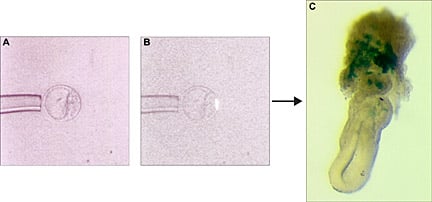
Clonal analysis of cell fate. Labeled polar trophectoderm cell from a blastocyst (B) and its progeny at E6.5. Clonal descendants (blue) are visible in the ectoplancental cone region of the embryo (C).