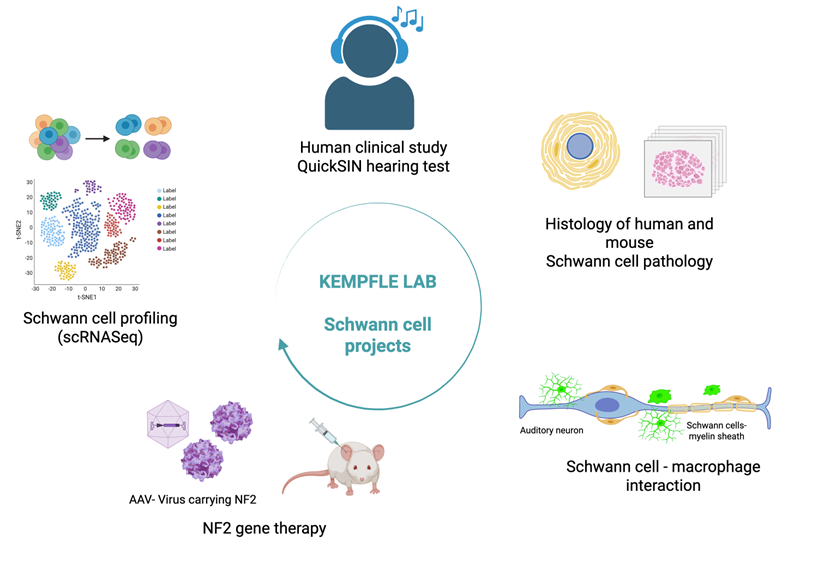
Projects
Scope of the Lab
My lab focuses on regenerative approaches to improve inner ear hearing loss, employing innovative techniques of cellular reprogramming, gene therapy and small molecule drug development. She has a special interest in Schwann cells and glia of the inner ear and the auditory system and their role in repair and hearing restoration.
Studying Schwann cells and non-neural cell types in disease and regeneration provides critical insights into the biology and progression of inner ear hearing loss and future treatment methods.
In Neurofibromatosis Type 2 (NF2), tumors arising from Schwann cells (vestibular schwannomas) lead to sensorineural hearing loss due to mutations in the NF2 gene encoding the tumor suppressor protein merlin. By understanding how merlin dysfunction drives schwannoma development and hearing loss, researchers can uncover common pathways involved in both hereditary and sporadic vestibular schwannomas. This knowledge can inform the development of targeted therapies, biomarkers, and improved management strategies for all patients with vestibular schwannomas, not just those with NF2.
What We Discovered
Our recent research in a mouse model of NF2 has shown something surprising: hearing loss begins long before tumors appear. The first signs are very subtle and happen at the level of synapses, tiny structures in the ear that connect sound-detecting hair cells to the auditory, hearing nerve cells that carry sound to the brain. We call this “hidden hearing loss,” because standard hearing tests can miss it at first.
- We also discovered that synaptopathy (loss of synapses) in NF2 may be caused by abnormal Schwann cells in the hearing portion of the inner ear, which help cover and protect nerves with a coating called myelin
- In addition, Macrophages, a type of immune cell that normally helps clean up damage, become overactive and might actually be making things worse.
Ongoing projects in the lab
We want to understand exactly how and when these cells cause hearing loss, and whether stopping or treating them could help preserve or recover hearing:
1. Track changes in the ear over time – We’ll study how nerve coating (myelin) breaks down and how immune cells become activated in young vs. older NF2 mice.
- Study cell behavior at the genetic level – Using advanced gene-reading tools, we’ll look at which genes are turned on or off in Schwann cells and immune cells during hearing loss.
- Test new treatment ideas – We’ll try turning off the immune cells to see if it slows or stops the hearing loss.
- All of this will help us identify better tests to track Schwann cell abnormalities and hearing loss in patients with NF2.
- Regeneration treatment for inner ear synapses for future targeted medicine – We are developing small molecule treatments for sustained inner ear delivery and treatment of sensorineural hearing loss.
Why It Matters
This line of study could lead to the first treatments aimed at preventing or regenerating hearing loss in patients with inner ear hearing loss related to Schwann cells and immune cells. Early treatment could help patients keep their hearing and improve their quality of life.
Significance
Treatment and detection of subclinical hearing loss will help to
- Develop therapies that protect nerve function before it's too late
- Offer personalized treatments based on how each patient’s [DG1] Schwann cells and immune cells behave
What’s Next?
We are building a new roadmap for hearing loss in NF2 and novel regeneration strategies for sensorineural hearing loss—from prevention to precision medicine. This research not only aims to preserve hearing, but also to open the door to new therapies for other nerve diseases involving Schwann cells and inflammation.