Chromosome silencing to study Down Syndrome
XIST transgenes can silence an autosome
The X-linked human XIST gene produces a large, non-coding RNA, which we showed is expressed exclusively from the inactive X chromosome (Xi) and accumulates to coat the X chromosome in cis. XIST RNA on the Xi induces a series of chromatin modifications that collectively silence the chromosome. Subsequent work from our lab and others indicates XIST RNA is also capable of silencing autosomal chromosomes.
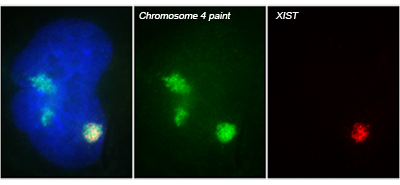
Translating dosage compensation to trisomy 21
Using genome editing with zinc finger nucleases (and currently CRISPR/Cas9), we targeted a large, inducible XIST transgene into the one of three Chromosome 21s in induced pluripotent stem cells (iPSCs) derived from Down syndrome patient cells. XIST RNA coats the extra chromosome 21 and triggers chromosome-wide transcriptional silencing and stable heterochromatin modifications, to form a “Chromosome 21 Barr Body.”
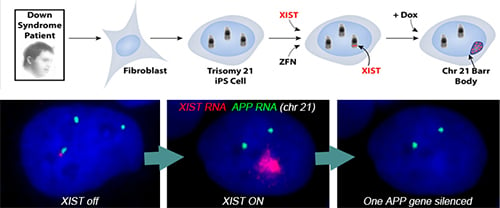
Translating dosage compensation to trisomy 21. Nature. 2013 Aug 15;500(7462):296-300.
Trisomy silencing normalizes Down syndrome hematopoietic defects
Trisomy 21 confers approximately 500-fold greater incidence of acute megakaryocytic leukemia (AMKL) and a ~20-fold greater risk for acute lymphoblastic leukemia (ALL). In addition, less severe hematopoietic abnormalities are present in most individuals with DS, including immune system defects.
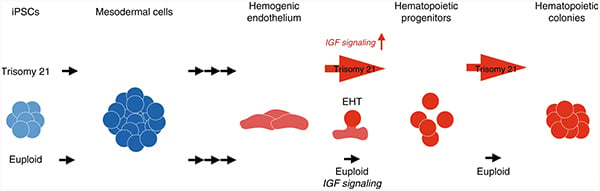
We have demonstrated that trisomy silencing by XIST-induced expression from one chromosome 21 can correct the regulatory mechanisms that underlie the hematopoietic cell pathologies of DS. Trisomy silencing reduced the overproduction of megakaryocyte and erythrocyte colonies in multiple transgenic clones. This also normalized an excess of CD43+ hematopoietic progenitors but did not impact earlier CD34+/CD43− population. Overall, results support that XIST normalized the hematopoietic process by rebalancing dosage-sensitive chromosome 21 genes.
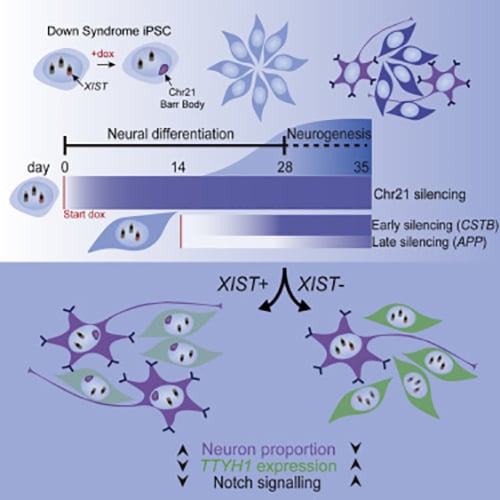
Silencing Trisomy 21 Promotes Neuronal Differentiation
We have demonstrated that XIST mediated chromosome silencing occurs in differentiated cells, contrary to the expectation that pluripotency factors are required.
The cell pathologies that underlie cognitive disabilities in Down syndrome are not well understood. We have investigated the effects of trisomy 21 on in vitro DS neurodevelopment, combining bulk and single-cell RNA sequencing (scRNA-seq) with molecular cytology of otherwise identical cells, with and without XIST-mediated dosage compensation induced from pluripotency or later in differentiation. This study demonstrated that over-expression of chr21 genes confers a developmental delay in terminal differentiation of neural stem cells (NSCs) to neurons.
Trisomy 21 causes deficits in angiogenesis and Notch signaling
To understand which cell types and pathways are more directly affected by trisomy 21 (T21), we used an inducible-XIST system to silence one chromosome 21 in vitro.
T21 caused the dysregulation of Notch signaling in iPSCs, potentially affecting cell-type programming. Further analyses identified dysregulation of pathways important for two cell types: neurogenesis and angiogenesis.
An in vitro assay for microvasculature formation revealed a cellular pathology involving delayed tube formation in response to angiogenic signals. Parallel transcriptomic analysis of endothelia further showed deficits in angiogenesis regulators.
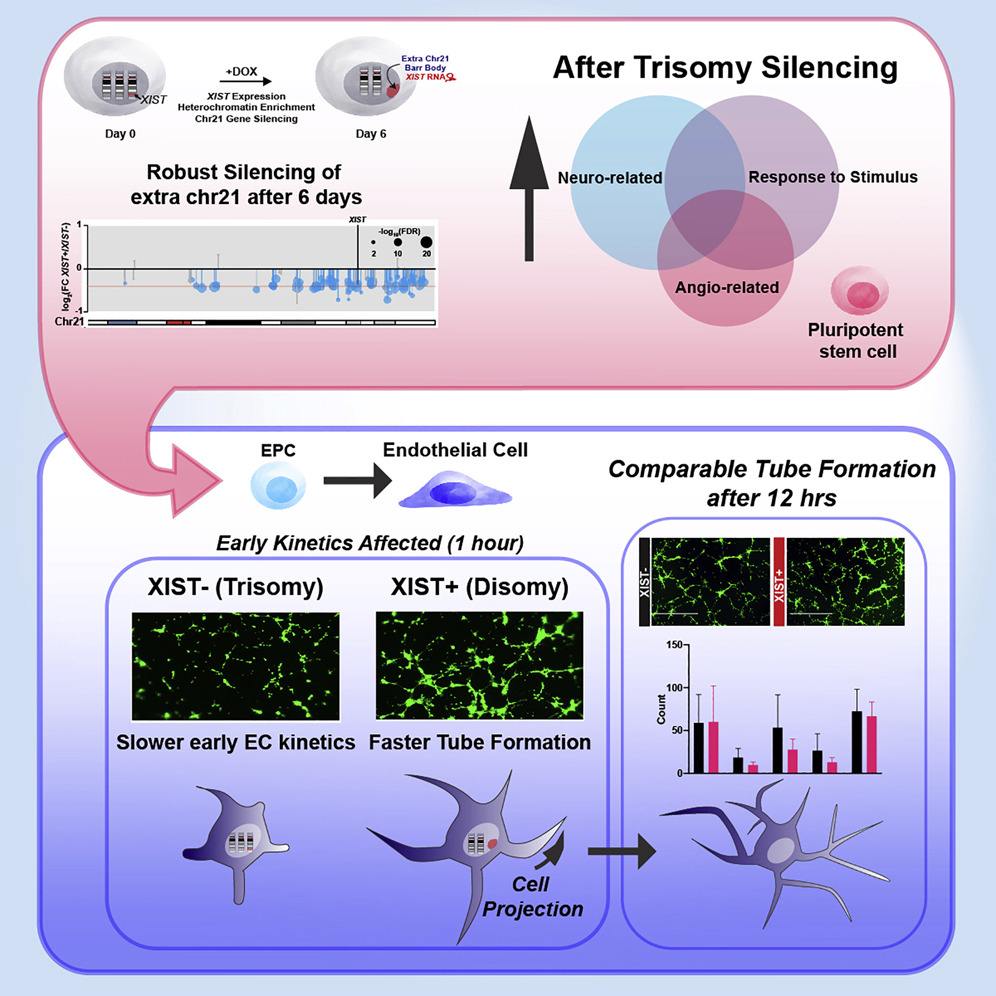
Chromosome silencing in vitro reveals trisomy 21 causes cell-autonomous deficits in angiogenesis and early dysregulation in Notch signaling. Cell Rep. 2022 Aug 9;40(6):111174.
