Behavioral Analysis
Behavior is the output of the nervous system and automated worm tracking systems are a powerful tool to probe the genetic basis of behavior. The worm trackers we use come in several different flavors, each with their own strengths.
The multi worm tacker
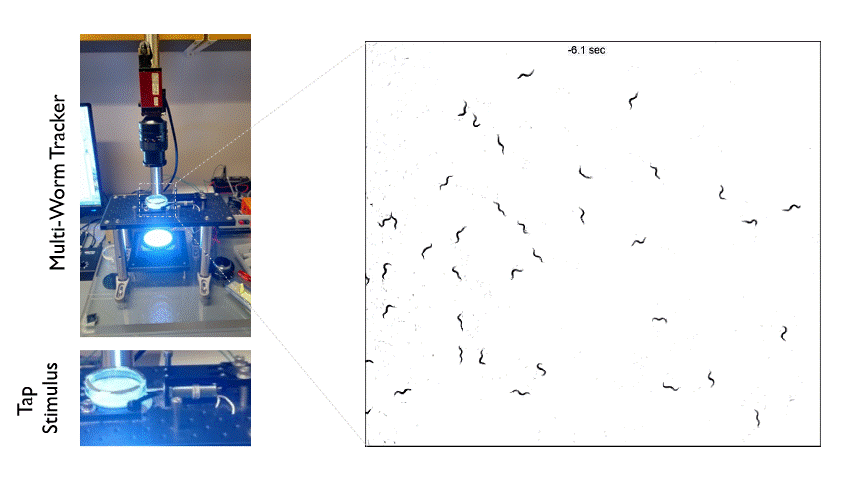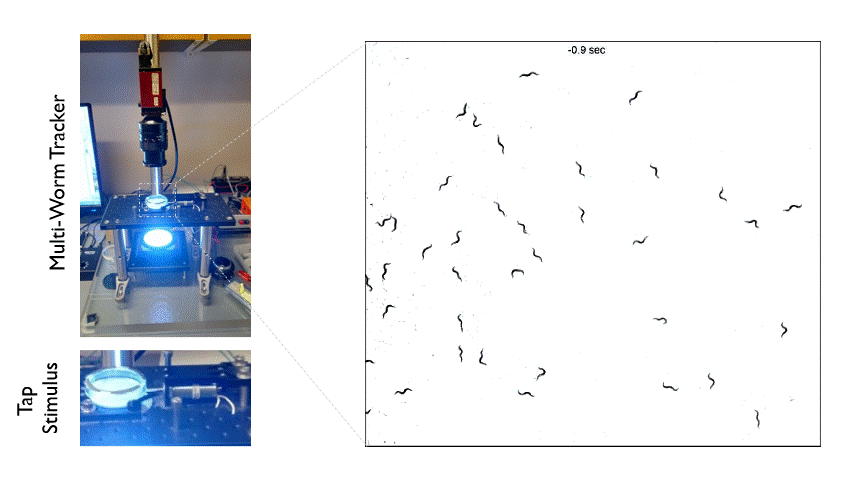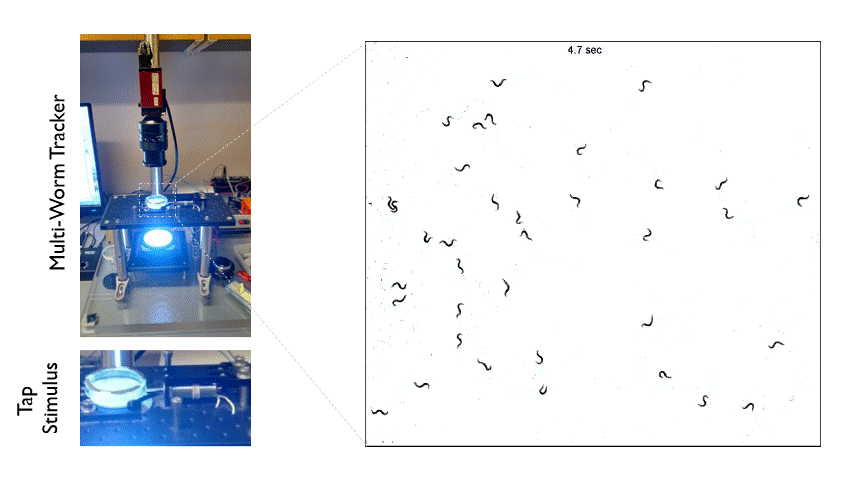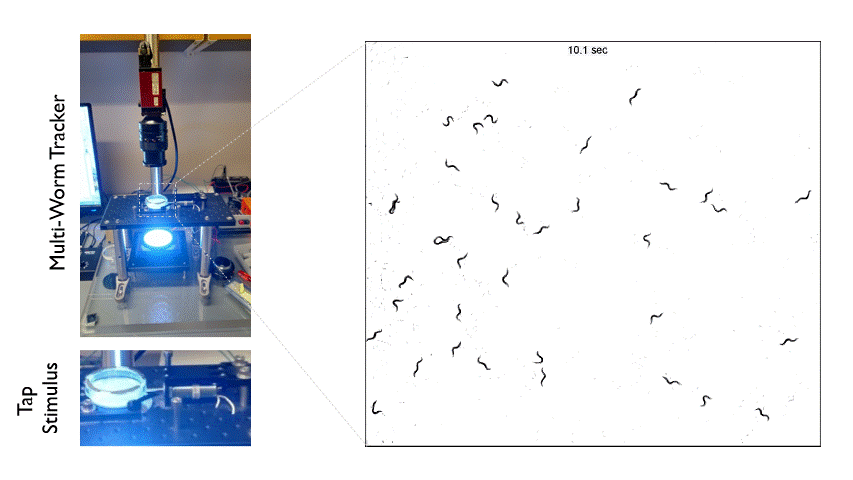
The Multi-Worm Tracker consists of an imaging rig and tracking software that allows high throughput behavioral analysis at a population level. Above is an example of how we can use the Multi-Worm Tracker to study the escape response in a population of worms by delivering a mechanical tap stimulus to the side of a petri-dish containing many animals.
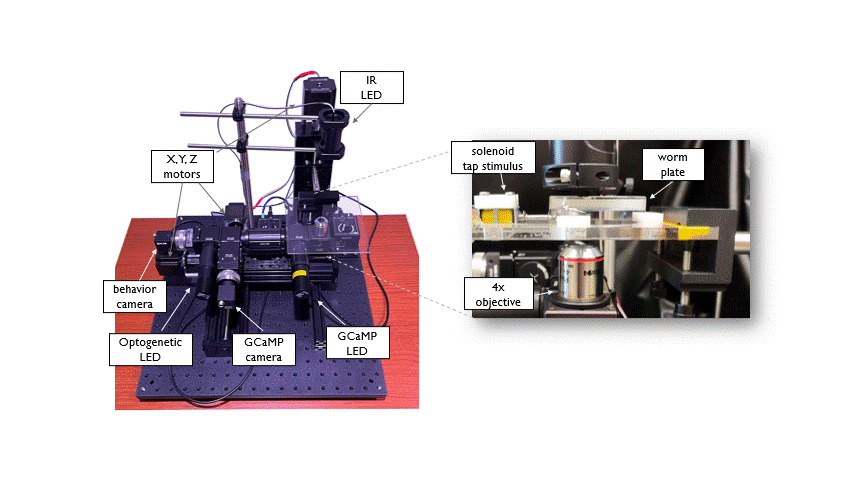
The Open Auto Scope is a motorized single-worm tracking microscope that is used for high resolution, long term imaging of individual C. elegans. The system is equiped with two optical paths (IR and GFP) to acquire simultaneous recordings of behavior and neuronal activity in freely moving animals. Integrated Optogenetic LEDs allow manipulation of neuronal activity. A tracking algorithm identifies the worm in real time and sends commands to the motors to keep the animal positioned under the microscope objective as it crawls across a petri dish. This allows long duration (hours to days) recordings of individuals. This system was designed and built in collaboration with the Venkatachalam lab at Northeastern University.
Calcium Imaging
Calcium influx is a key mediator of neuronal excitation, particularly in C. elegans which lack voltage gated sodium channels. genetically encoded calcium indicators allow researchers to monitor neuronal activity in intact behaving animals. GCaMP is a modified green fluorescent protein (GFP) which is linked to the calcium binding domain of calmodulin. Calcium influx results in a conformational change in the GCaMP protein increasing the fluorescent output of the GFP allowing real time monitoring of neuronal activity.
Below is an example of calcium imaging of the RIM neurons in freely behaving animals. A tap stimulus triggers the escape response and activates the RIM neurons. The left side of the video shows the GCaMP fluorescence and the animals behavior is shown on the right.
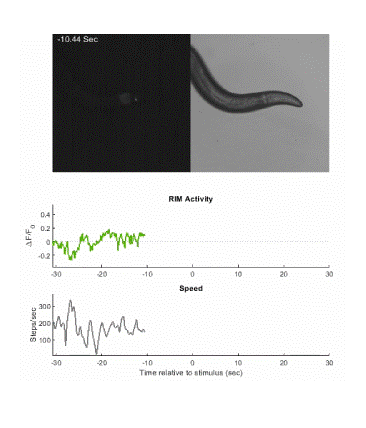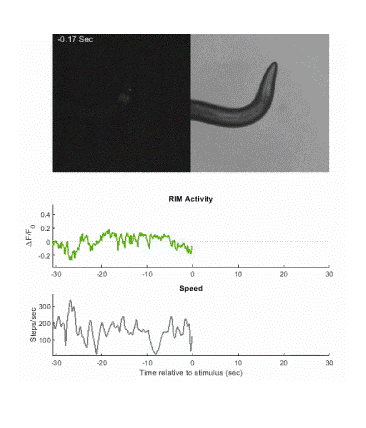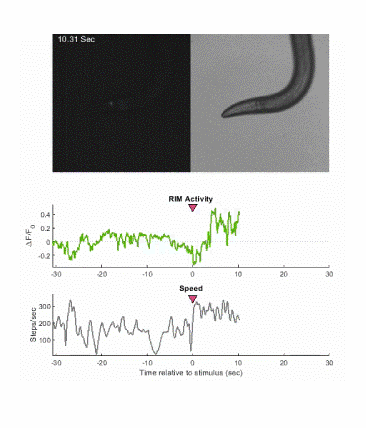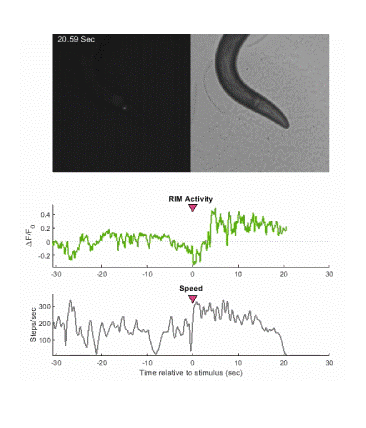
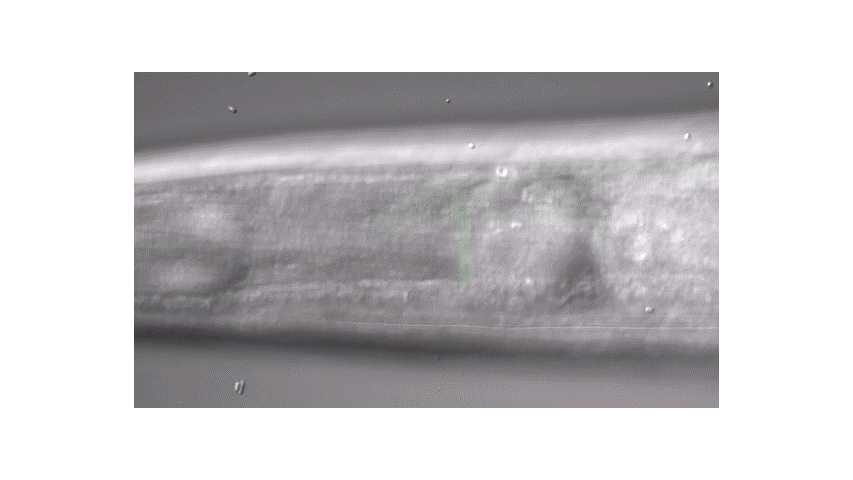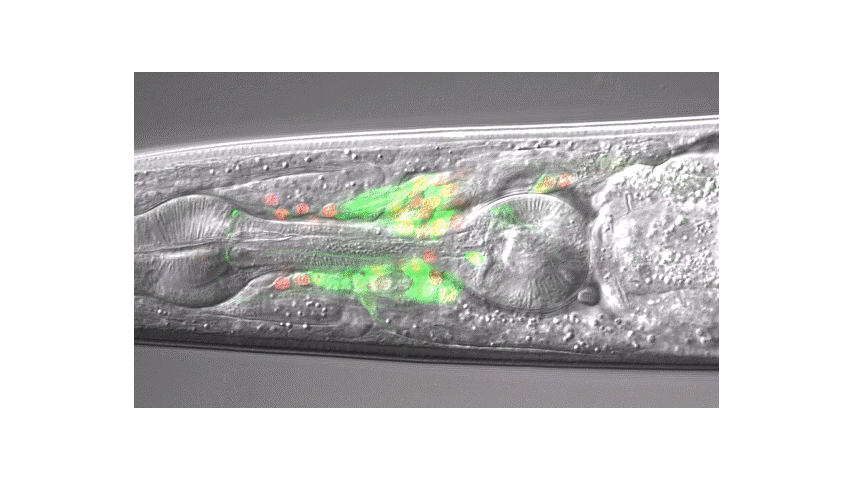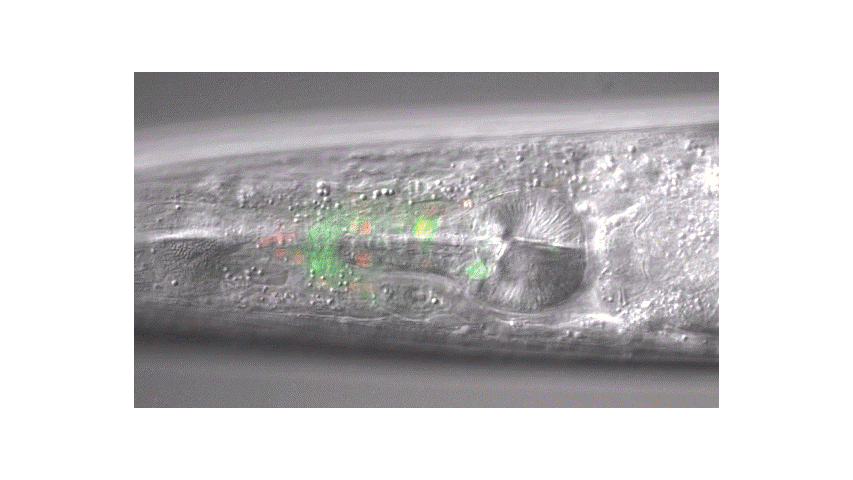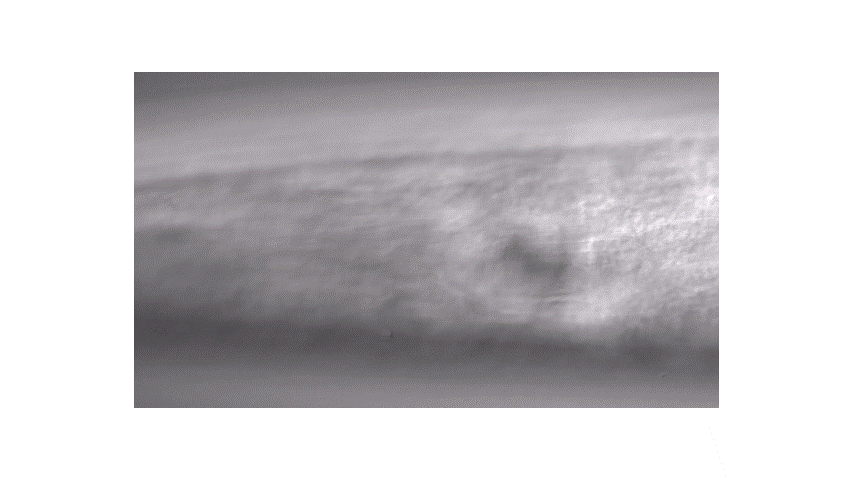
Calcium imaging in one or a few neurons is by far the most common approach but there is increasing adoption of "whole brain" imaging in C. elegans. Below is a confocal microscope stack of an animal expressing GCaMP6 under a pan-neuronal promoter. Red fluorescent protein (RFP) marks the nuclei of each neuron which aids in cell identification and tracking.
Optogenetics
Transgenic expression of light gated ion channels in neurons has revolutionized the field of neuroscience, allowing researchers to turn on and off neurons with different wavelengths of light. These optogenetic techniques are powerful tools to dissect the function of neurons and neural circuits in intact behaving animals. C. elegans is particularly well suited to optogenetic manipulation due to its optical transparency and a variety of cell specific promoters.
In the video above, animals express the light gated cation channel ChannelRhodopsin in a pair of motor neurons called the RIV. These neurons innervate ventral neck muscle and are important for the initiation of the omega turn during the C. elegans escape response. Shining blue light on the animal causes the RIV to become active and induces a ventral turn. Techniques like this allow us to determine the function of individual neurons in the coordination of complex behavior.
