HIV Protease
 |
|
HIV-1 Protease-substrate complex (1KJ4) |
 |
|
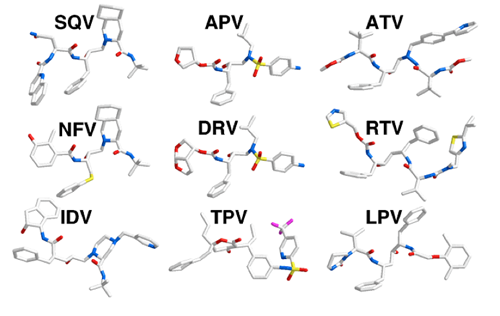
FDA-approved HIV-1 protease inhibitors |
HIV-1 protease, a 99 residue, homodimeric aspartyl protease, processes the viral polyproteins at several cleavage sites. The active site of the enzyme is located at the dimeric interface. X-ray crystallographic studies from our group on the protease-substrate complexes revealed a consensus substrate binding mode, describing the substrate envelope. (Prabu-Jeyabalan, et.al., 2002)
Nonfunctional protease results in immature, noninfectious virions, therefore protease inhibitors are among the major antiviral drugs in the treatment of HIV-1-infected patients. These highly potent drugs have significantly increased the life span of the infected individuals. However, drug resistance is a major reason for the lack of a permanent cure for AIDS.
The nine FDA approved HIV-1 protease inhibitors were developed with extensive use of structure-based drug design. The atomic details of inhibitor binding are well characterized allowing a structural understanding the molecular basis for drug resistance in HIV-1 protease. (King, et.al., 2004, Ali, et.al., 2010)
Selected mutations in response to protease inhibitor treatment have altered the shape of the active site, potentially altered the dynamics (Foulkes-Murzycki, et.al., 2007) and even altered the sequence of the cleavage sites in the Gag polyprotein. (Kolli, et.al., 2006, Kolli, et.al., 2009)
All of these interdependent changes act in synergy to confer drug resistance while simultaneously maintaining the fitness of the virus.
New strategies, such as incorporation of the substrate envelope constraint to design robust inhibitors that are active against even highly resistant viral variants and decrease the probability of emerging drug resistance, are necessary to continue to effectively target this key protein in HIV-1 life cycle. (Chellappan, et.al., 2007, Reddy, et.al., 2007, Nalam & Schiffer, 2008)

