Mutations in VPS45
During our studies of SNARE regulation, we also investigated the endosomal Sec1/Munc18 homolog, VPS45 and dissected how it regulates SNARE complex assembly and membrane fusion.
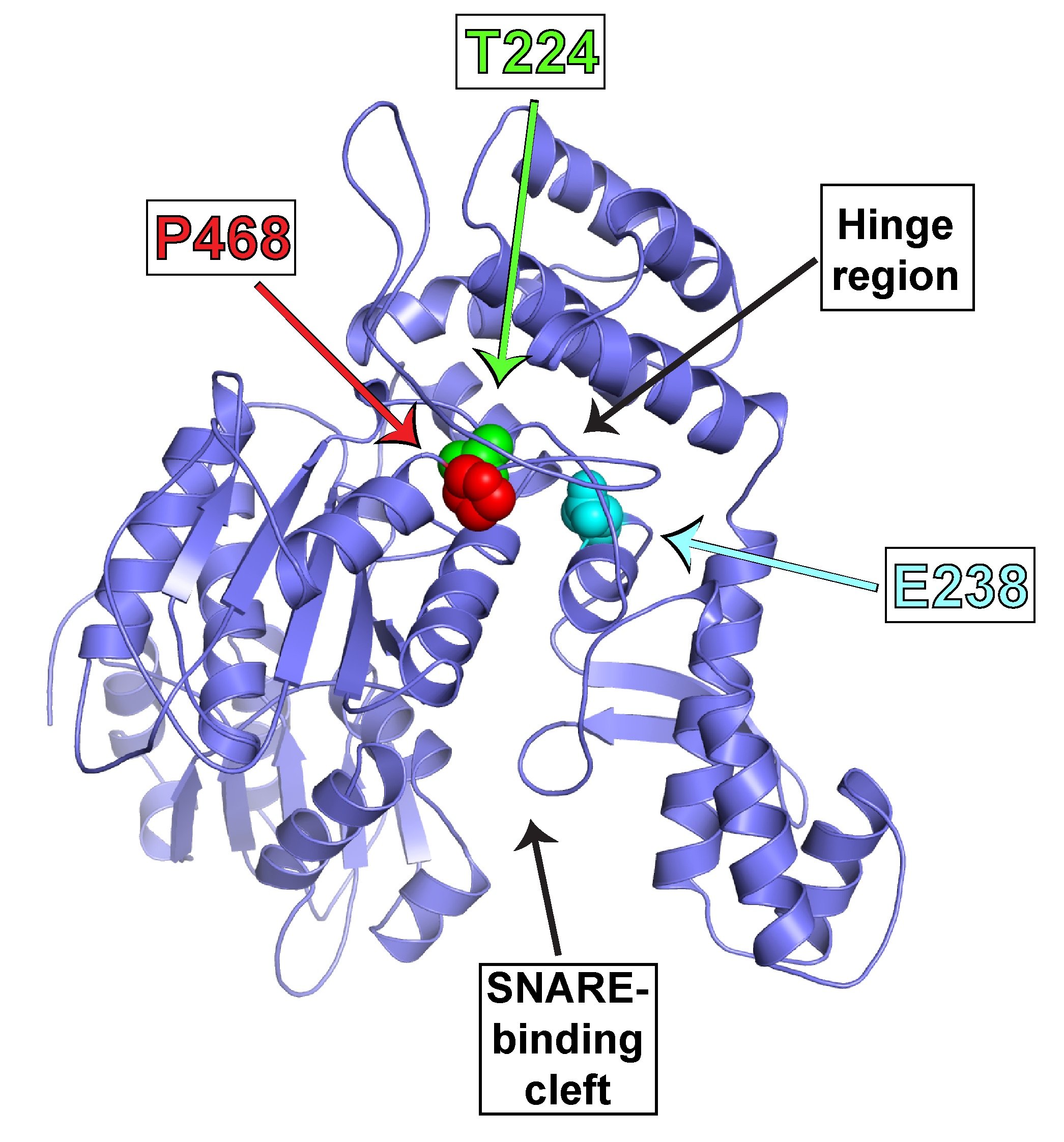
We are now applying our biochemical insights to understanding the human VPS45 and how dysfunctional point mutations in VPS45 lead to severe congenital neutropenia, bone marrow defects, and myelofibrosis in children. We are using a variety of techniques, in collaboration with Peter Newburger’s lab at UMass Chan to elucidate the mechanism behind this phenotype. These include biochemistry and structural biology methods to probe the effect of the mutations on the structure of VPS45 and its ability to interact with known binding partners; in complementary studies, cell biological techniques and fluorescence microscopy are used to understand the effect of the VPS45 mutations on cellular trafficking and organelle biogenesis. Mutant cells and mouse models are being developed using CRISPR/Cas9 technologies.