The Exocyst Complex
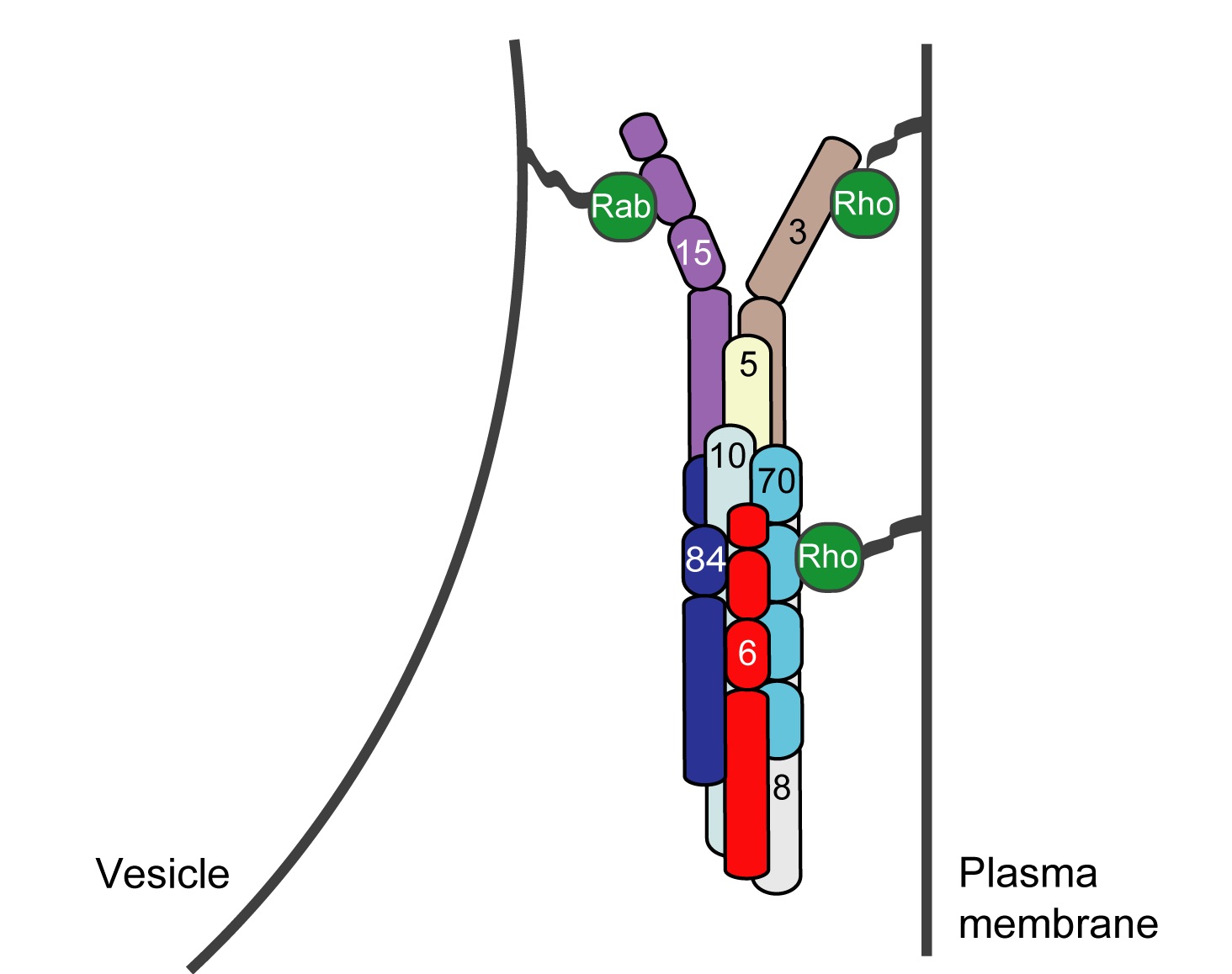
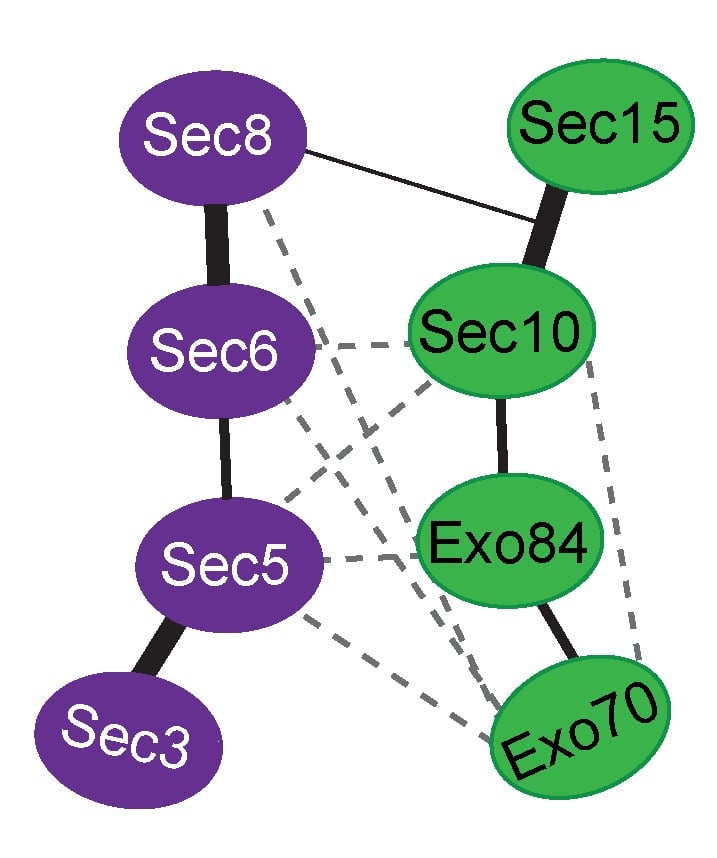
Exocytosis is the regulated fusion of secretory vesicles at the plasma membrane, to promote cell growth, division, and intercellular communication. We study how SNARE-mediated fusion of transport vesicles at the plasma membrane is temporally and spatially controlled by the exocyst, a large multisubunit tethering complex, in collaboration with the SNARE-regulator Sec1. To answer these questions, we use biochemical and structural methods, combined with examination of specific mutant phenotypes in yeast using microscopy and secretion assays. We discovered the first direct interaction between the exocyst and SNARE proteins, and recently mapped interactions between them. Also, we identified residues on the subunit Sec6 that are essential for proper localization of exocyst to sites of exocytosis. Furthermore, with several excellent collaborators, we examined direct interactions of exocyst with the myosin motor that carries secretory vesicles. We determined the crystal structure of the exocyst subunit Sec6, and showed that it is structurally homologous to other exocyst subunits (with <10% sequence identity), and members of other tethering complexes. Furthermore, we modeled the structures of the other subunits, and predicted that they also have similar helical bundle structures. Our recent work demonstrates our groundbreaking purification method for intact yeast exocyst complexes, our genetic and biochemical dissection of the architecture of the exocyst, and the first view of the overall structure of the complex using negative stain EM (Heider et al., 2016) in collaboration with Mike Rout’s lab at Rockefeller and Adam Frost’s lab at UCSF. Our goal is to answer many essential questions about how the exocyst functions in the cell--what does it look like? How does it bind to its partners, including the Rab GTPase Sec4? How is it localized? How does it tether vesicles? How does it function with Sec1 to control the SNARE fusion machinery? Current research focuses on higher resolution structural studies as well as development of two in vitro TIRF-based single molecule assays to directly examine the roles of exocyst in a) tethering (with Jeff Gelles at Brandeis Univ) and b) SNARE-mediated fusion of vesicles (with Tae-Young Yoon at Seoul National Univ, South Korea).