Division of Biologic Laboratories in the Early 20th Century
On July 7, 1914, the State Department of Public Health was created, replacing the old Board of Health. The divisions first organized included: Administration, Sanitary Engineering, Water and Sewage Laboratories, Food and Drugs, Communicable Diseases, and Hygiene. Additionally, all the laboratories, including the Antitoxin and Vaccine Laboratory and Diagnostic Laboratory were organized into the Division of Biologic Laboratories.

in Hinton test at
Wassermann Lab.
From Commonhealth
p21. 1954.
In 1915 the Wassermann Laboratory, under Dr. W. A. Hinton as Assistant Director, was established as a unit of the Division of Biologic Laboratories. The Wassermann Laboratory had originally been established through the Department of Neuro-Pathology at the Harvard Medical School for the purposes of using Wassermann tests, an antibody test for syphilis, to diagnose mental illness. The laboratory oversight and functions were transferred from Harvard to the State Department of Public Health when Dr. Allen J. McLaughlin, the first Massachusetts Commissioner of Public Health, sponsored legislation whereby free tests for syphilis were made available to all citizens of Massachusetts. The Wassermann Laboratory remained part of the Division of Biologic Laboratories until 1944.24
Dr. Smith resigned as director in 1914 to join the Rockefeller Institute and the directorship was filled by Milton J. Rosenau, MD,, Professor of Preventive Medicine at Harvard Medical School and formerly Director of the Hygienic Laboratory, U.S. Public Health Service. Rosenau carried the laboratory through six difficult years, from the outbreak of World War I, with its critical shortages of personnel and supply, into the post war period of growth.25 Rosenau served as Director of the newly formed Division of Biologic Laboratories until 1920 when Dr. Benjamin White, formerly with the New York City Laboratories became the first full-time director of the Division of Biologic Laboratories.
Dr. Rosenau applied to the Public Health Service in 1916 for a Federal License to ship biologic products in interstate commerce. Federal License No. 64 was first granted in 1917 for diphtheria antitoxin, vaccine virus, and bacterial vaccine from the typhoid bacillus. There have been many subsequent amendments for additional products and reissues for name changes. Dr. Rosenau did not initially recognize licensure as being significant, but it has proved to have far-reaching consequences in the subsequent history of the Laboratories. The legal privilege of selling surplus products out-of state provided an opportunity for the Laboratory to make a small profit. The state was also then able to dispose of excess supplies of biologics to other areas in need. If less concrete, a greater significance to licensure was the relationship the Laboratory formed with the Hygienic Laboratory (later the National Institute of Health). This allowed the Antitoxin and Vaccine Laboratory to gain immeasurable advice, knowledge, assistance, and increase in standards for all aspects of biologics production. Under the direction of Dr. White, the privileges and implementation of the Federal License was formally recognized when in 1921 the Massachusetts Legislature authorized the sale of surplus products.26

issued to the Antitoxin
and Vaccine Laboratory,
Department of Public
Health, Commonwealth
of Massachusetts dated
July 27, 1923.
(reissue after name
change of original
1917 license)

issued to the
Massachusetts Public
Health Biologic
Laboratories, dated
August 20, 1996.
(reissue after name
change of original
1917 license)
Diphtheria antitoxin remained an important component of the Laboratories’ research and products. Demonstrations of the Schick test, which was used to determine some immunity to diphtheria and the need for vaccination, began in 1915 and distribution of Schick outfits commenced in 1917. The Schick test was based on the work of Bela Schick, who developed the test to determine if a person had some immunity to diphtheria. He reasoned that the injection of a very small amount of diphtheria toxin under the skin would produce a reddening and slight swelling at the site. If such a reaction occurred, it indicated that the patient had not previously been exposed to diphtheria. A lack of reddening of the injection area indicated that the patient had previously been exposed to diphtheria and therefore had immunity to it. Having already developed immunity eliminated the need for treatment, saving precious antitoxin for those without immunity.27

Rosenau, Bela Schick.
1923.
Additional advancement occurred in the 1920s and 1930s under the direction of Dr. White. Standards within the laboratory began to conform more closely to those of the National Institute of Health (originally known as the Hygienic Laboratory, but changed to the National Institute of Health in 1930) and there were significant improvements in technical equipment. Refinements in production methods included the ‘washing’ of typhoid vaccine which reduced the number of unfavorable reactions. Other investigative activity resulted in improved methods of bleeding horses and improved methods of producing smallpox vaccine. Work on antiserum against pneumococcal bacteria was of particular note. A 1931 state wide pneumonia study was undertaken after refinement of pneumonia serum and accumulating evidence for its clinical usefulness. Significant quantities of refined type I and II pneumonia serum were prepared and distributed through depots statewide. The results of the Pneumonia Control Study, lasting through 1935, showed that the case fatality rate for most of the commoner types of pneumonia could be cut in half by means of adequate serum therapy.28

“Captain”
produced enough
Antitoxin to
protect 86,000.
From the early days of the Antitoxin and Vaccine Laboratory, teaching was considered an important part of its functions. Dr. Smith and Dr. Rosenau brought their students from Harvard Medical School to the Laboratory for instruction, even though there was no specific course offered in laboratory immunology. The first scheduled courses given at the Laboratory began in 1923 in conjunction with the recently founded Harvard School of Public Health. This first course, Applied Immunology, was taught by Dr. White and Dr. Elliot Robinson.
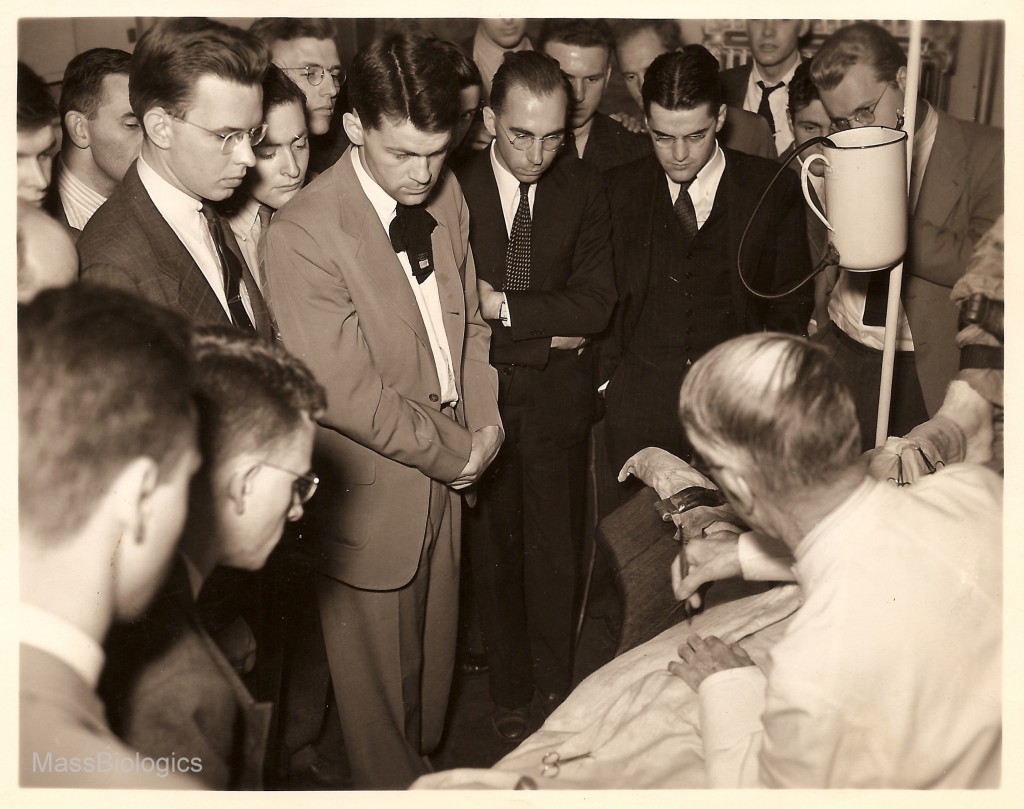
vaccination on calf
to medical students.
Soon after, arrangements were made with other schools, such as Simmons College, for the training of students in practical public health laboratory methods. Each of the early directors also served on the faculty of Harvard Medical School, playing valuable roles in medical education, but also shedding light on problems of public health for other faculty and medical professionals. Collaboration with Harvard Medical School also led to a series of new biologic products. Work with scientists from the Department of Bacteriology led to a new serum for the treatment of meningitis in 1927 and an anti-typhus serum in 1932.29
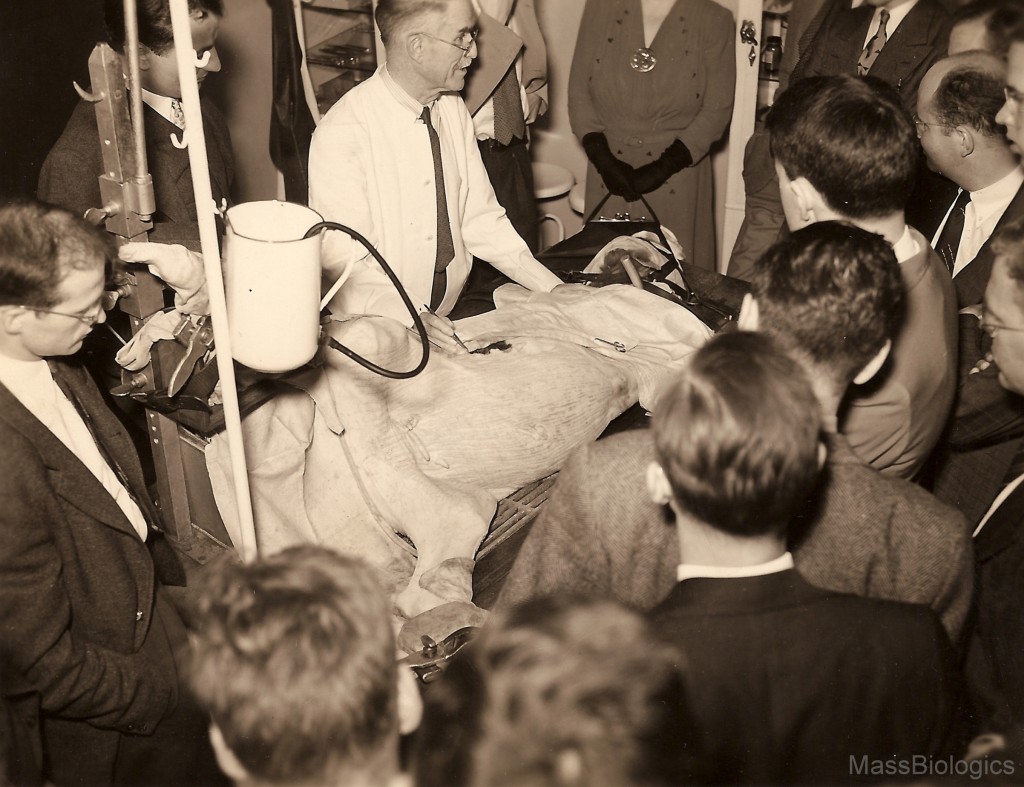
vaccination on calf
to medical students.
In 1934 Dr. White resigned because of ill health and Elliot S. Robinson, MD,, Ph.D., then Assistant Director, became Director. Under Dr. Robinson’s direction noteworthy advancements were made in achieving high yields of diphtheria toxin by production using defined pepton-free media. As well, an emphasis was placed on pneumonia serum studies. Significantly, a new source of antibodies, derived from human tissues, and free from the antigenic reaction of a foreign source, was developed by Dr. Charles F. McKhann of the Harvard Medical School. He found a placental extract for the prevention and modification of measles. These antibodies, produced and distributed from 1934 to 1944, were the first products derived from human blood to be distributed by the laboratory.30