Research Areas
Endosomal TLRs and SLE.
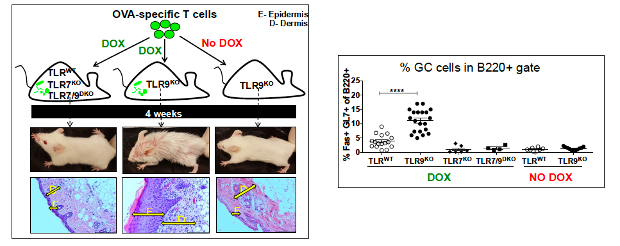
Endosomal TLRs play a major role in lupus pathogenesis and the activation of autoreactive B cells. To further define the specific roles of TLR9 and TLR7, we use mice that express a transgene-encoded BCR that recognizes autologous IgG2a. B cells from these mice can be activated by immune complexes that incorporate nucleic acids and can be used to monitor downstream events that regulate plasma cell differentiation. We have also developed a model of cutaneous lupus induced by the adoptive transfer of T cells specific for a pseudo-autoantigen. Skin lesions in these mice are dependent on the expression of TLR7 but exacerbated by TLR9-deficiency. Ongoing studies are exploring how endosomal TLRs modulate disease severity.
Nucleic Acid Receptors and Monogenic Autoinflammation.
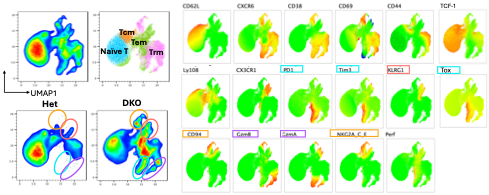
GOF mutations in STING, a key component of the cytosolic DNA sensing system, lead to the development of an autoinflammatory syndrome referred to as SAVI. SAVI patients can develop severe vasculitis and debilitating pulmonary complications. We have generated murine models of SAVI that recapitulate many of the clinical manifestations exhibited by SAVI patients, including interstitial lung disease and ulcerating skin lesions. Current studies, in collaboration with Dr. Kate Fitzgerald (Innate Immunity) and Mehdi Rashighi (Dermatology), are focused on delineation of the critical cell types and mechanisms responsible for lung inflammation and cutaneous disorders in these SAVI mice, with a particular focus on T cell effector subsets.
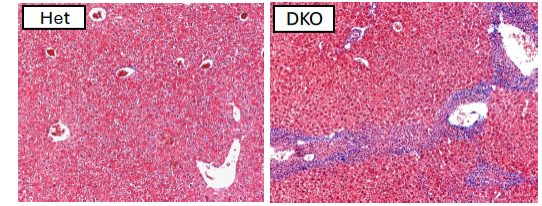
We also study mice deficient for DNaseII, a nuclease that plays a key role in the endolysosomal degradation of apoptotic debris. While DNaseII deficiency alone is embryonic lethal, loss of the type I IFN receptor rescues these mice. The DNAseII x IFNaR double-deficient mice develop a systemic autoimmune disease associated with hepatic inflammation and fibrosis. Remarkably, patients with DNAseII hypomorphic mutation also develop liver complications. We are using this model to elucidate mechanisms responsible for autoimmune hepatitis.
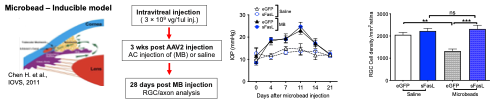
Interplay of Membrane-bound and Soluble FasL in Ocular Pathology.
Membrane-bound (mFasL) is a potent inducer of both apoptosis and inflammation depending on the nature of the Fas-expressing target cell. It is therefore a potentially dangerous molecule that requires stringent regulation. One mechanism that limits the activity of mFasL is metalloproteinase cleavage of the extracellular domain and the release of a soluble fragment (sFasL) that opposes the activity of mFasL. FasL is constitutively expressed in healthy eyes where is it also constitutively cleaved. However, during ocular pathologies such as glaucoma or retinal detachment, FasL is no longer cleaved and the accumulation of mFasL plays a key role in the destruction of the target tissues. We are now trying to understand how FasL cleavage is regulated and how sFasL promotes neuroprotection. We are also working with Drs. Guangping Gao and Phillip Tai (Gene Therapy, UMass Chan) and Dr. Meredith Gregory-Ksander (Schepens Eye Institute) to develop AAV vectors that deliver sFasL to the retina for the treatment of glaucoma.