Mammalian SWI/SNF Enzymes as Drivers of Cancer
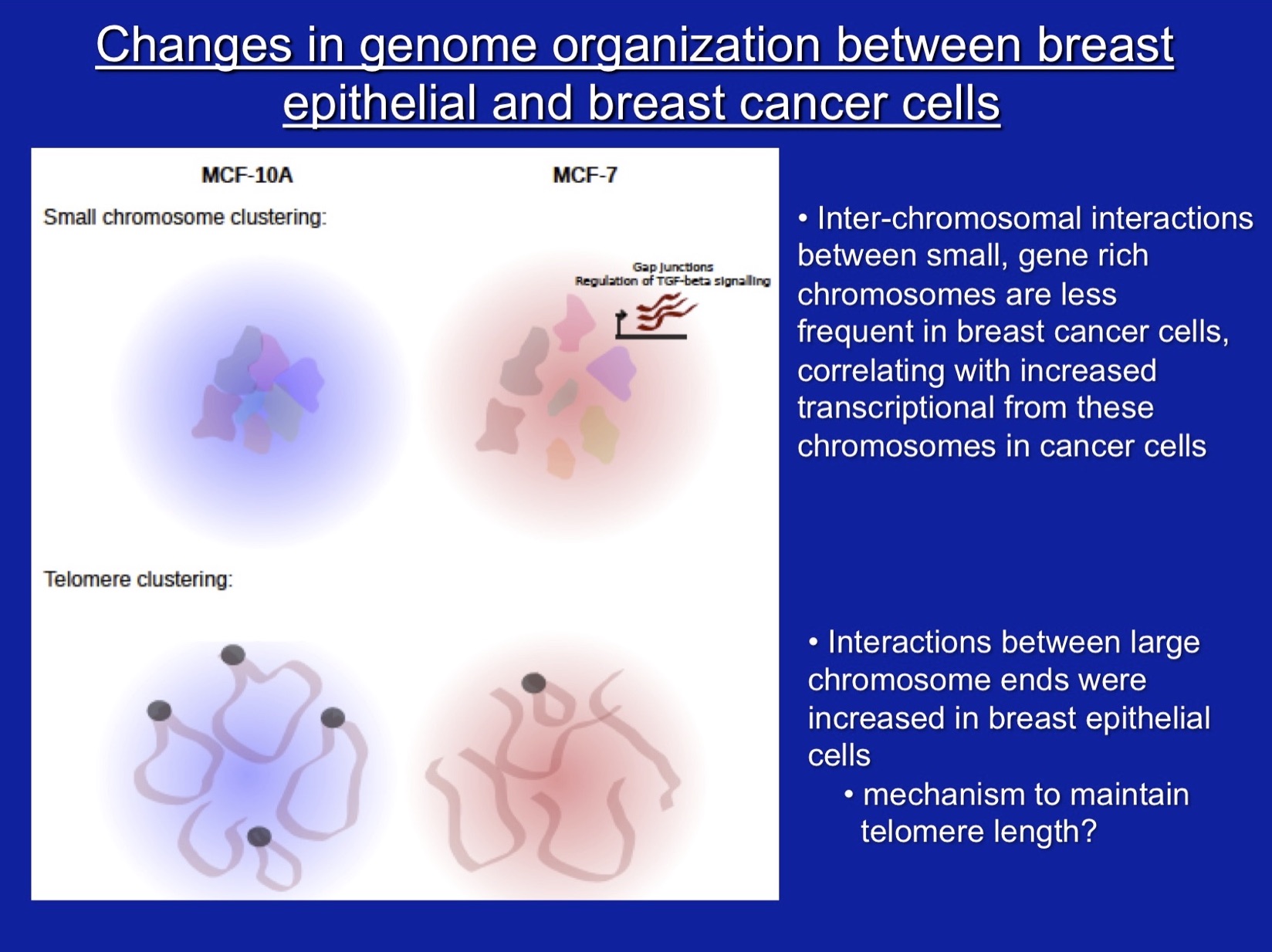
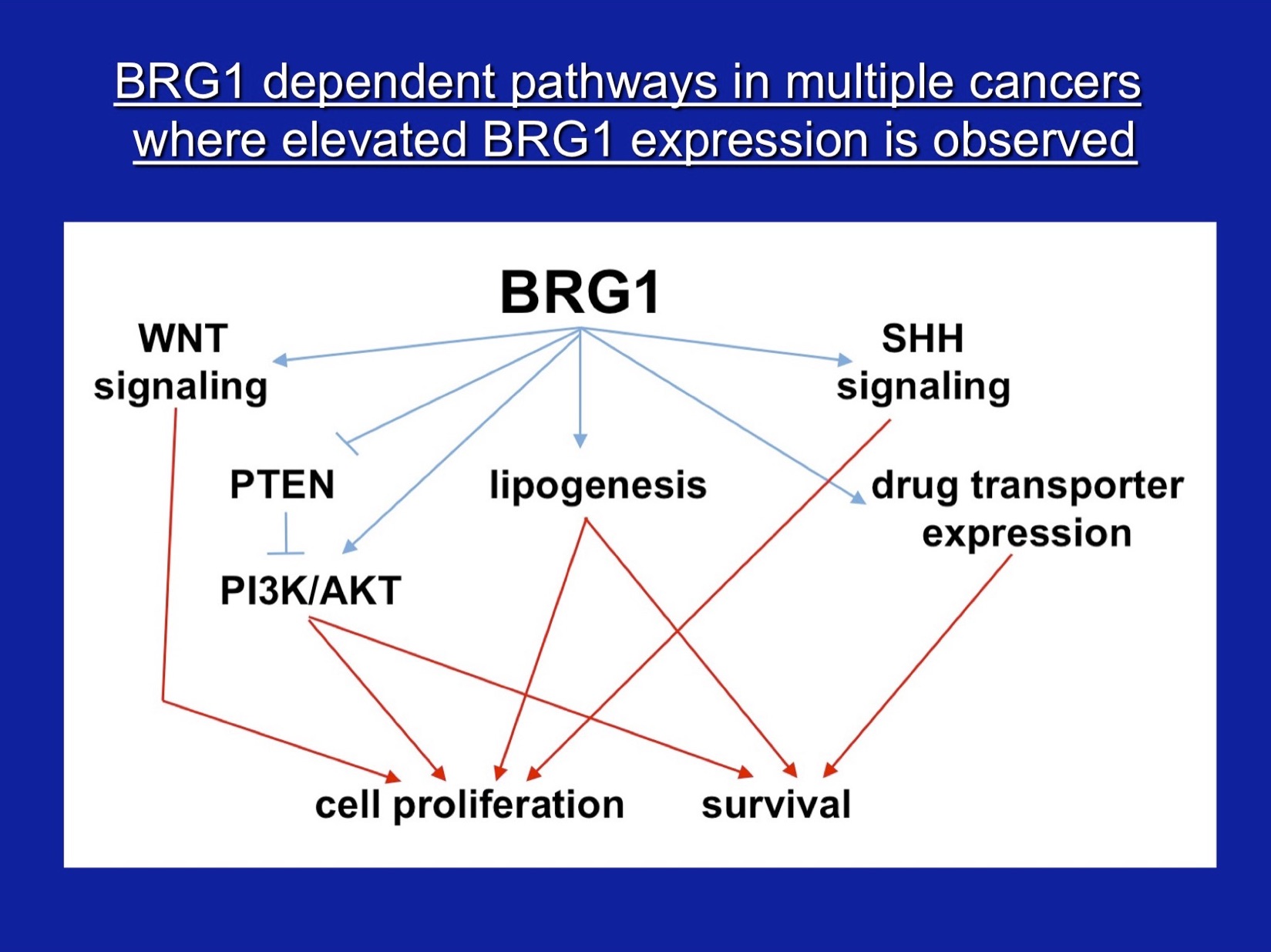
Our other major interest is the role of SWI/SNF subunit proteins in cell proliferation and cancer. For these studies, we have characterized properties of the SWI/SNF subunits, Ini1, Brg1, and Brm, in vivo, in culture, and in vitro. Our work demonstrated that the Ini1 subunit is a bona fide tumor suppressor and that there is a strong compensatory mechanism that upregulates Ini1 production in cells possessing only one functional copy of the gene. More recent work, nearly all of which has been in collaboration with Jeffrey Nickerson’s lab, has focused on Brg1 and Brm, the mutually exclusive ATPase subunits that drive mammalian SWI/SNF enzymatic functions. Work on the Brg1 ATPase demonstrated that functional Brg1 is required to maintain proper nuclear shape and structure, marking Brg1 as one of the few known nuclear factors that contribute to the structural integrity of the cell nucleus. Brg1 also maintains the integrity of higher-order chromatin structure in breast epithelial cells. Brg1 was originally characterized as a breast cancer ‘tumor suppressor’, however, we showed that Brg1 and Brm are required for breast epithelial as well as breast cancer cell proliferation and that nearly all human breast tumors, regardless of subtype, show overexpression of both proteins. Current work is focused on characterizing functional differences in how Brg1 and Brm regulate epithelial and cancer cell proliferation. We have established that Brg1 functions in the regulation of lipid synthesis and glucose metabolism in breast cancer but not breast epithelial cells. Brg1 also plays an important role in the establishment of cellular chemoresistance to conventional chemotherapy approaches by contributing to drug-induced upregulation of ABC transporters, which increase drug efflux. Efforts to move our work from basic to translatable research have resulted proof of principle findings that small molecule inhibitors of Brg1 block increased lipid synthesis typical of breast cancer cells without affecting lipid synthesis in epithelial cells and also block drug-induced upregulation of ABC transporters, thereby sensitizing cells to conventional chemotherapeutic drugs. We propose that Brg1 inhibitors are potential therapeutic agents for breast cancer therapy.